The Role of Aardvarks (Orycteropus Afer)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evidence from Fossil Crustaceans in Cold-Water C
Klompmaker et al. BMC Evolutionary Biology (2016) 16:132 DOI 10.1186/s12862-016-0694-0 RESEARCH ARTICLE Open Access Evolution of body size, vision, and biodiversity of coral-associated organisms: evidence from fossil crustaceans in cold- water coral and tropical coral ecosystems Adiël A. Klompmaker1,2,3*, Sten L. Jakobsen4 and Bodil W. Lauridsen5 Abstract Background: Modern cold-water coral and tropical coral environments harbor a highly diverse and ecologically important macrofauna of crustaceans that face elevated extinction risks due to reef decline. The effect of environmental conditions acting on decapod crustaceans comparing these two habitats is poorly understood today and in deep time. Here, we compare the biodiversity, eye socket height as a proxy for eye size, and body size of decapods in fossil cold-water and tropical reefs that formed prior to human disturbance. Results: We show that decapod biodiversity is higher in fossiltropicalreefsfromTheNetherlands,Italy,and Spain compared to that of the exceptionally well-preserved Paleocene (Danian) cold-water reef/mound ecosystem from Faxe (Denmark), where decapod diversity is highest in a more heterogeneous, mixed bryozoan-coral habitat instead of in coral and bryozoan-dominated facies. The relatively low diversity at Faxe was not influenced substantially by the preceding Cretaceous/Paleogene extinction event that is not apparent in the standing diversity of decapods in our analyses, or by sampling, preservation, and/or a latitudinal diversity gradient. Instead, the lower availability of food and fewer hiding places for decapods may explain this low diversity. Furthermore, decapods from Faxe are larger than those from tropical waters for half of the comparisons, which may be caused by a lower number of predators, the delayed maturity, and the increased life span of crustaceans in deeper, colder waters. -

Freshwater Fishes
WESTERN CAPE PROVINCE state oF BIODIVERSITY 2007 TABLE OF CONTENTS Chapter 1 Introduction 2 Chapter 2 Methods 17 Chapter 3 Freshwater fishes 18 Chapter 4 Amphibians 36 Chapter 5 Reptiles 55 Chapter 6 Mammals 75 Chapter 7 Avifauna 89 Chapter 8 Flora & Vegetation 112 Chapter 9 Land and Protected Areas 139 Chapter 10 Status of River Health 159 Cover page photographs by Andrew Turner (CapeNature), Roger Bills (SAIAB) & Wicus Leeuwner. ISBN 978-0-620-39289-1 SCIENTIFIC SERVICES 2 Western Cape Province State of Biodiversity 2007 CHAPTER 1 INTRODUCTION Andrew Turner [email protected] 1 “We live at a historic moment, a time in which the world’s biological diversity is being rapidly destroyed. The present geological period has more species than any other, yet the current rate of extinction of species is greater now than at any time in the past. Ecosystems and communities are being degraded and destroyed, and species are being driven to extinction. The species that persist are losing genetic variation as the number of individuals in populations shrinks, unique populations and subspecies are destroyed, and remaining populations become increasingly isolated from one another. The cause of this loss of biological diversity at all levels is the range of human activity that alters and destroys natural habitats to suit human needs.” (Primack, 2002). CapeNature launched its State of Biodiversity Programme (SoBP) to assess and monitor the state of biodiversity in the Western Cape in 1999. This programme delivered its first report in 2002 and these reports are updated every five years. The current report (2007) reports on the changes to the state of vertebrate biodiversity and land under conservation usage. -

Classification of the Apidae (Hymenoptera)
Utah State University DigitalCommons@USU Mi Bee Lab 9-21-1990 Classification of the Apidae (Hymenoptera) Charles D. Michener University of Kansas Follow this and additional works at: https://digitalcommons.usu.edu/bee_lab_mi Part of the Entomology Commons Recommended Citation Michener, Charles D., "Classification of the Apidae (Hymenoptera)" (1990). Mi. Paper 153. https://digitalcommons.usu.edu/bee_lab_mi/153 This Article is brought to you for free and open access by the Bee Lab at DigitalCommons@USU. It has been accepted for inclusion in Mi by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. 4 WWvyvlrWryrXvW-WvWrW^^ I • • •_ ••^«_«).•>.• •.*.« THE UNIVERSITY OF KANSAS SCIENC5;^ULLETIN LIBRARY Vol. 54, No. 4, pp. 75-164 Sept. 21,1990 OCT 23 1990 HARVARD Classification of the Apidae^ (Hymenoptera) BY Charles D. Michener'^ Appendix: Trigona genalis Friese, a Hitherto Unplaced New Guinea Species BY Charles D. Michener and Shoichi F. Sakagami'^ CONTENTS Abstract 76 Introduction 76 Terminology and Materials 77 Analysis of Relationships among Apid Subfamilies 79 Key to the Subfamilies of Apidae 84 Subfamily Meliponinae 84 Description, 84; Larva, 85; Nest, 85; Social Behavior, 85; Distribution, 85 Relationships among Meliponine Genera 85 History, 85; Analysis, 86; Biogeography, 96; Behavior, 97; Labial palpi, 99; Wing venation, 99; Male genitalia, 102; Poison glands, 103; Chromosome numbers, 103; Convergence, 104; Classificatory questions, 104 Fossil Meliponinae 105 Meliponorytes, -
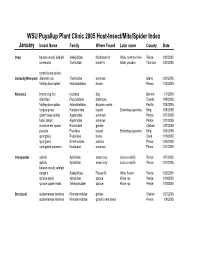
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

A Systematic Study of Berkheya and Allies (Compositae)
A systematic study of Berkheya and allies (Compositae) A thesis submitted in the fulfilment of the requirements for the degree of Master of Science of Rhodes University by Ntombifikile Phaliso April 2013 Supervisor: Prof. N.P. Barker (Botany Department, Rhodes University) Co-supervisor: Dr. Robert McKenzie (Botany Department, Rhodes University) Table of contents: Title ……………………………………………………………………………..I Acknowledgements…………………………………………………………...III Declaration……………………………………………………………………IV Abstract…………………………………………………………………………1 Chapter 1: General Introduction……………………………………………..3 Chapter 2: The molecular phylogeny of Berkheya and allies……………...12 Aims………………………………………………………………………………………….12 2.1: Molecular (DNA-based) systematic……………………………………………………..12 2.2: Methods and Materials…………………………………………………………………..18 2.1.1: Sampling…………………………………………………………………………..18 2.1.2: DNA extraction, amplification and sequencing…………………………………..18 2.1.3: Sequence alignment……………………………………………………………..19 2.1.4: Phylogenetic Analyses …………………………………………………………...21 2.3: Results…………………………………………………………………………………..22 2.3.1: ITS data set………………………………………………………………………..22 2.3.2: psbA-trnH data set………………………………………………………………..23 2.3.3: Combined data set………………………………………………………………...24 2.4: Discussion……………………………………………………………………………….28 2.4.1: Phylogenetic relationships within the Berkheya clade……………………………28 2.4.2: Insights from the psbA-trnH & combined data set phylogenies………………….37 2.4.3: Taxonomic implications: paraphyly of Berkheya………………………………...39 2.4.4: Taxonomic Implications: Correspondence with -

A Preliminary Detective Survey of Hymenopteran Insects at Jazan Lake Dam Region, Southwest of Saudi Arabia
Saudi Journal of Biological Sciences 28 (2021) 2342–2351 Contents lists available at ScienceDirect Saudi Journal of Biological Sciences journal homepage: www.sciencedirect.com Original article A preliminary detective survey of hymenopteran insects at Jazan Lake Dam Region, Southwest of Saudi Arabia Hanan Abo El-Kassem Bosly 1 Biology Department - Faculty of Science - Jazan University, Saudi Arabia article info abstract Article history: A preliminary detective survey for the hymenopteran insect fauna of Jazan Lake dam region, Southwest Received 16 November 2020 Saudi Arabia, was carried out for one year from January 2018 to January 2019 using mainly sweep nets Revised 6 January 2021 and Malaise traps. The survey revealed the presence of three hymenopteran Superfamilies (Apoidea, Accepted 12 January 2021 Vespoidea and Evanioidea) representing 15 species belonging to 10 genera of 6 families (Apidae, Available online 28 January 2021 Crabronidae, Sphecidae, Vespidae, Mutillidae, and Evaniidae). The largest number of species has belonged to the family Crabronidae is represented by 6 species under 2 genera. While the family Apidae, is repre- Keywords: sented by 2 species under 2 genera. Family Vespidae is represented by 2 species of one genus. While, the Survey rest of the families Sphecidae, Mutillida, and Evaniidae each is represented by only one species and one Insect fauna Hymenoptera genus each. Eleven species are predators, two species are pollinators and two species are parasitics. Note Jazan for each family was provided, and species was provided with synonyms and general and taxonomic Saudi Arabia remarks and their worldwide geographic distribution and information about their economic importance are also included. -

Host-Plant Genotypic Diversity Mediates the Distribution of an Ecosystem Engineer
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Supervised Undergraduate Student Research Chancellor’s Honors Program Projects and Creative Work Spring 4-2006 Genotypic diversity mediates the distribution of an ecosystem engineer Kerri Margaret Crawford University of Tennessee-Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_chanhonoproj Recommended Citation Crawford, Kerri Margaret, "Genotypic diversity mediates the distribution of an ecosystem engineer" (2006). Chancellor’s Honors Program Projects. https://trace.tennessee.edu/utk_chanhonoproj/949 This is brought to you for free and open access by the Supervised Undergraduate Student Research and Creative Work at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Chancellor’s Honors Program Projects by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. • f" .1' I,'r· ... 4 ....., ' 1 Genotypic diversity mediates the distribution of an ecosystem engineer 2 3 4 5 6 7 Kerri M. Crawfordl, Gregory M. Crutsinger, and Nathan J. Sanders2 8 9 10 11 Department 0/Ecology and Evolutionary Biology, University o/Tennessee, Knoxville, Tennessee 12 37996 13 14 lAuthor for correspondence: email: [email protected]. phone: (865) 974-2976,/ax: (865) 974 15 3067 16 2Senior thesis advisor 17 18 19 20 21 22 23 24 25 26 27 28 29 30 12 April 2006 1 1 Abstract 2 Ecosystem engineers physically modify environments, but much remains to be learned about 3 both their effects on community structure and the factors that predict their occurrence. In this 4 study, we used experiments and observations to examine the effects of the bunch galling midge, 5 Rhopalomyia solidaginis, on arthropod species associated with Solidago altissima. -

Biodiversity and Ecology of Critically Endangered, Rûens Silcrete Renosterveld in the Buffeljagsrivier Area, Swellendam
Biodiversity and Ecology of Critically Endangered, Rûens Silcrete Renosterveld in the Buffeljagsrivier area, Swellendam by Johannes Philippus Groenewald Thesis presented in fulfilment of the requirements for the degree of Masters in Science in Conservation Ecology in the Faculty of AgriSciences at Stellenbosch University Supervisor: Prof. Michael J. Samways Co-supervisor: Dr. Ruan Veldtman December 2014 Stellenbosch University http://scholar.sun.ac.za Declaration I hereby declare that the work contained in this thesis, for the degree of Master of Science in Conservation Ecology, is my own work that have not been previously published in full or in part at any other University. All work that are not my own, are acknowledge in the thesis. ___________________ Date: ____________ Groenewald J.P. Copyright © 2014 Stellenbosch University All rights reserved ii Stellenbosch University http://scholar.sun.ac.za Acknowledgements Firstly I want to thank my supervisor Prof. M. J. Samways for his guidance and patience through the years and my co-supervisor Dr. R. Veldtman for his help the past few years. This project would not have been possible without the help of Prof. H. Geertsema, who helped me with the identification of the Lepidoptera and other insect caught in the study area. Also want to thank Dr. K. Oberlander for the help with the identification of the Oxalis species found in the study area and Flora Cameron from CREW with the identification of some of the special plants growing in the area. I further express my gratitude to Dr. Odette Curtis from the Overberg Renosterveld Project, who helped with the identification of the rare species found in the study area as well as information about grazing and burning of Renosterveld. -

Follow-Up Visits to Alatash – Dinder Lion Conservation Unit Ethiopia
Follow-up visits to Alatash – Dinder Lion Conservation Unit Ethiopia & Sudan Hans Bauer, Ameer Awad, Eyob Sitotaw and Claudio Sillero-Zubiri 1-20 March 2017, Alatash National Park, Ethiopia 30 April - 16 May 2017, Dinder National Park, Sudan Report published in Oxford, September 2017 Wildlife Conservation Research Unit - University of Oxford (WildCRU); Ethiopian Wolf Conservation Programme (EWCP); Ethiopian Wildlife Conservation Authority (EWCA); Mekele University (MU); Sudan Wildlife Research Centre (SWRC). Funded by the Born Free Foundation and Born Free USA. 1 Contents Summary ................................................................................................................................................. 3 Teams ...................................................................................................................................................... 4 Introduction ............................................................................................................................................ 5 Methods .................................................................................................................................................. 5 Area description - Alatash ....................................................................................................................... 6 Area description - Dinder ........................................................................................................................ 7 Results - Alatash ..................................................................................................................................... -

Afrotherian Conservation – Number 16
AFROTHERIAN CONSERVATION Newsletter of the IUCN/SSC Afrotheria Specialist Group Number 16 Edited by PJ Stephenson September 2020 Afrotherian Conservation is published annually by the measure the effectiveness of SSC’s actions on biodiversity IUCN Species Survival Commission Afrotheria Specialist conservation, identification of major new initiatives Group to promote the exchange of news and information needed to address critical conservation issues, on the conservation of, and applied research into, consultations on developing policies, guidelines and aardvarks, golden moles, hyraxes, otter shrews, sengis and standards, and increasing visibility and public awareness of tenrecs. the work of SSC, its network and key partners. Remarkably, 2020 marks the end of the current IUCN Published by IUCN, Gland, Switzerland. quadrennium, which means we will be dissolving the © 2020 International Union for Conservation of Nature membership once again in early 2021, then reassembling it and Natural Resources based on feedback from our members. I will be in touch ISSN: 1664-6754 with all members at the relevant time to find out who wishes to remain a member and whether there are any Find out more about the Group people you feel should be added to our group. No one is on our website at http://afrotheria.net/ASG.html automatically re-admitted, however, so you will all need to and on Twitter @Tweeting_Tenrec actively inform me of your wishes. We will very likely need to reassess the conservation status of all our species during the next quadrennium, so get ready for another round of Red Listing starting Message from the Chair sometime in the not too distant future. -

Birding Tour to Ghana Specializing on Upper Guinea Forest 12–26 January 2018
Birding Tour to Ghana Specializing on Upper Guinea Forest 12–26 January 2018 Chocolate-backed Kingfisher, Ankasa Resource Reserve (Dan Casey photo) Participants: Jim Brown (Missoula, MT) Dan Casey (Billings and Somers, MT) Steve Feiner (Portland, OR) Bob & Carolyn Jones (Billings, MT) Diane Kook (Bend, OR) Judy Meredith (Bend, OR) Leaders: Paul Mensah, Jackson Owusu, & Jeff Marks Prepared by Jeff Marks Executive Director, Montana Bird Advocacy Birding Ghana, Montana Bird Advocacy, January 2018, Page 1 Tour Summary Our trip spanned latitudes from about 5° to 9.5°N and longitudes from about 3°W to the prime meridian. Weather was characterized by high cloud cover and haze, in part from Harmattan winds that blow from the northeast and carry particulates from the Sahara Desert. Temperatures were relatively pleasant as a result, and precipitation was almost nonexistent. Everyone stayed healthy, the AC on the bus functioned perfectly, the tropical fruits (i.e., bananas, mangos, papayas, and pineapples) that Paul and Jackson obtained from roadside sellers were exquisite and perfectly ripe, the meals and lodgings were passable, and the jokes from Jeff tolerable, for the most part. We detected 380 species of birds, including some that were heard but not seen. We did especially well with kingfishers, bee-eaters, greenbuls, and sunbirds. We observed 28 species of diurnal raptors, which is not a large number for this part of the world, but everyone was happy with the wonderful looks we obtained of species such as African Harrier-Hawk, African Cuckoo-Hawk, Hooded Vulture, White-headed Vulture, Bat Hawk (pair at nest!), Long-tailed Hawk, Red-chested Goshawk, Grasshopper Buzzard, African Hobby, and Lanner Falcon. -

The Gambia: a Taste of Africa, November 2017
Tropical Birding - Trip Report The Gambia: A Taste of Africa, November 2017 A Tropical Birding “Chilled” SET DEPARTURE tour The Gambia A Taste of Africa Just Six Hours Away From The UK November 2017 TOUR LEADERS: Alan Davies and Iain Campbell Report by Alan Davies Photos by Iain Campbell Egyptian Plover. The main target for most people on the tour www.tropicalbirding.com +1-409-515-9110 [email protected] p.1 Tropical Birding - Trip Report The Gambia: A Taste of Africa, November 2017 Red-throated Bee-eaters We arrived in the capital of The Gambia, Banjul, early evening just as the light was fading. Our flight in from the UK was delayed so no time for any real birding on this first day of our “Chilled Birding Tour”. Our local guide Tijan and our ground crew met us at the airport. We piled into Tijan’s well used minibus as Little Swifts and Yellow-billed Kites flew above us. A short drive took us to our lovely small boutique hotel complete with pool and lovely private gardens, we were going to enjoy staying here. Having settled in we all met up for a pre-dinner drink in the warmth of an African evening. The food was delicious, and we chatted excitedly about the birds that lay ahead on this nine- day trip to The Gambia, the first time in West Africa for all our guests. At first light we were exploring the gardens of the hotel and enjoying the warmth after leaving the chilly UK behind. Both Red-eyed and Laughing Doves were easy to see and a flash of colour announced the arrival of our first Beautiful Sunbird, this tiny gem certainly lived up to its name! A bird flew in landing in a fig tree and again our jaws dropped, a Yellow-crowned Gonolek what a beauty! Shocking red below, black above with a daffodil yellow crown, we were loving Gambian birds already.