Analysis of the Bactericidal Activity of Naive Rabbit Serum Against Staphylococcus Aureus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LOST "Raised by Another" (YELLOW) 9/23/04
LOST “Raised by Another” CAST LIST BOONE................................Ian Somerhalder CHARLIE..............................Dominic Monaghan CLAIRE...............................Emilie de Ravin HURLEY...............................Jorge Garcia JACK.................................Matthew Fox JIN..................................Daniel Dae Kim KATE.................................Evangeline Lilly LOCKE................................Terry O’Quinn MICHAEL..............................Harold Perrineau SAWYER...............................Josh Holloway SAYID................................Naveen Andrews SHANNON..............................Maggie Grace SUN..................................Yunjin Kim WALT.................................Malcolm David Kelley THOMAS............................... RACHEL............................... MALKIN............................... ETHAN................................ SLAVITT.............................. ARLENE............................... SCOTT................................ * STEVE................................ * www.pressexecute.com LOST "Raised by Another" (YELLOW) 9/23/04 LOST “Raised by Another” SET LIST INTERIORS THE VALLEY - Late Afternoon/Sunset CLAIRE’S CUBBY - Night/Dusk/Day ENTRANCE * ROCK WALL - Dusk/Night/Day * INFIRMARY CAVE - Morning JACK’S CAVE - Night * LOFT - Day - FLASHBACK MALKIN’S HOUSE - Day - FLASHBACK BEDROOM - Night - FLASHBACK LAW OFFICES CONFERENCE ROOM - Day - FLASHBACK EXTERIORS JUNGLE - Night/Day ELSEWHERE - Day CLEARING - Day BEACH - Day OPEN JUNGLE - Morning * SAWYER’S -

September 2010 Cinemann Table of Contents
September 2010 Cinemann Table of Contents 4 Summer Inception Movie Recap 6 Letter From the Editors 14 The Big C 8 Summer Television 15 Rent 10 The Emmy Awards Cinemann 2 Cinemann: Volume VI, Issue 1 Editors in Chief )--.#,"&0(1*,&./- 5.6&/,7$&,68(4/,&0/-! Andrew Demas !"#$%&!'#()*+%,-!.&,,%(#!'/%-! 9#(/&3!:*%,,&+&$/-!21%,&! 0)&!"&,$#1-! '/#+3&,-!;#<%=!>&*&+,$&%1-! Maggie Reinfeld .#()?!>+#(@6#1-!2?%A#%3! Senior Editors 2&"33(4/,&0/- B+&&1?#*6-!:&11&$$!C&33&+-! Matt Taub 23%(&!4#+#1$)-!5#6!4)++&,-! D)#/!E#+A*3%,-!.#F!G#3&H Alexandra Saali '/#+3&,!5/&++-!766#!58&($&+ @#+-!5#<#11#/!56%$/-!9#H (/&3!5%6&+@#H56%$/-!C&1+F! !"#$%&'()*+,-./ I#+=&+ !"#$%&'()&**"+ Letter From the EditorsDear Reader, Welcome back!! We are so thrilled to present you with WKHÀUVWLVVXHRICinemann,+RUDFH0DQQ·VÀOPDQGDUWV PDJD]LQH$V\RXVLIWWKURXJKWKHSDJHVZHKRSH\RX SOXQJHLQWRWKHMR\VRIWKLVVXPPHU·VPRYLHVDQGDUW ,QVLGH\RXZLOOÀQGDQDUUD\RIUHYLHZV%HJLQQLQJZLWK a look at summer’s highest grossing blockbusters, as ZHOODVLWVXQH[SHFWHGÁRSVZHZLOODOVREULQJ\RXXSWR GDWHZLWKUHYLHZVRIVXPPHUWHOHYLVLRQDQGWKHDWHUCin- emannGHOYHVLQWRKRZWKHLQQRYDWLYHSORWVRIWKHEULO- OLDQWO\FUDIWHG,QFHSWLRQDQGWKHUDXQFK\FRPHG\-HUVH\ 6KRUHKDYHWUDQVÀ[HGYLHZHUV&RPHG\VHHPHGWRUXOH at the 2010 Emmy Awards, and we are here to show you why. Inside we recount the night’s biggest moments and HYDOXDWHWKHGHFLVLRQEHKLQGWKHUHVXOWV:HURXQGRXW WKLVLVVXHZLWKDUHYLHZRI1HLO3DWULFN+DUULV·UHYLYDO RIRentDWWKH+ROO\ZRRG%RZOH[SORULQJWKHYHU\QD- WXUHRIWKHFKDOOHQJHVLQYROYHGLQUHVWDJLQJDFODVVLF:H hope you enjoy it. ,I\RXZRXOGOLNHWRZULWHIRU&LQHPDQQSOHDVHFRQWDFW -

JULIA CARTA Hair Stylist and Make-Up Artist
JULIA CARTA Hair Stylist and Make-Up Artist www.juliacarta.com PRESS JUNKETS/PUBLICITY EVENTS Matt Dillon - Grooming - WAYWARD PINES - London Press Junket Jeremy Priven - Grooming - BAFTA Awards - London Christian Bale - Grooming - AMERICAN HUSTLE - BAFTA Awards - London Naveen Andrews - Grooming - DIANA - London Press Junket Bruce Willis and Helen Mirren - Grooming - RED 2 - London Press Conference Ben Affleck - Grooming - ARGO - Sebastián Film Festival Press Junket Matthew Morrison - Grooming - WHAT TO EXPECT WHEN YOU’RE EXPECTING - London Press Junket Clark Gregg - Grooming - THE AVENGERS - London Press Junket Max Iron - Grooming - RED RIDING HOOD - London Press Junket and Premiere Mia Wasikowska - Hair - RESTLESS - Cannes Film Festival, Press Junket and Premiere Elle Fanning - Make-Up - SUPER 8 - London Press Junket Jamie Chung - Hair & Make-Up - SUCKERPUNCH - London Press Junket and Premiere Steve Carell - Grooming - DESPICABLE ME - London Press Junket and Premiere Mark Strong and Matthew Macfayden - Grooming - Cannes Film Festival, Press Junket and Premiere Michael C. Hall - Grooming - DEXTER - London Press Junket Jonah Hill - Grooming - GET HIM TO THE GREEK - London Press Junket and Premiere Laura Linney - Hair and Make-Up - THE BIG C - London Press Junket Ben Affleck - Grooming - THE TOWN - London and Dublin Press and Premiere Tour Andrew Lincoln - Grooming - THE WALKING DEAD - London Press Junket Rhys Ifans - Grooming - NANNY MCPHEE: THE BIG BANG (RETURNS) - London Press Junket and Premiere Bruce Willis - Grooming - RED - London -

V26 N5 2015 112 Page Layout.Indd
SeaAndBeScene BY STEPHANIE BEAUMONT 1 2 3 Nova Scotia stars in The Healer After countless stand-in roles for seaside states like Maine (Dolores Claiborne), Massachusetts (the Jesse Stone movies) and Washington (Christmas With Holly), Nova Scotia is finally taking the lead. “I immediately fell in love with the look and the feel of Nova Scotia, a perfect place for a magical, mystical tale,” says Paco Arango, writer/ director of The Healer which tells the story of a man who discovers he possesses the gift for healing people just as he arrives in the small town of Lunenburg, N.S. The Healer is a co-production with Academy Award-nominated 4 producer Enrique Posner and Michael Volpe of Halifax’s Topsail Productions (Trailer Park Boys, Mr. D). “We had an amazing team on this production, and we were thrilled to be up and running after a challenging start to 2015 for the Nova Scotia film industry,” Volpe shared. “The regulations for the province’s new Film Incentive Fund are now in place and people in the industry are actively engaged in trying to navigate the new system. We are cautiously optimistic about being able to continue to develop projects and to bring top-caliber filmmakers, like the international team on The Healer, to work in Nova Scotia.” The Healer stars Oliver Jackson-Cohen (Dracula), Camilla Luddington (Grey’s Anatomy), Jorge Garcia (Lost), Jonathan Pryce (Pirates of the 5 Caribbean) and Kaitlyn Bernard (Mom’s Day Away). 6 6 7 8 1. On location for The Healer, starring Oliver 6. Cast photo: (standing) Kaitlyn Bernard proceeds from this film are earmarked for the Jackson-Cohen (Alec), Kaitlyn Bernard (Abigail) (Abigail), (sitting) Oliver Jackson-Cohen (Alec), SeriousFun Children’s Network. -

Reminder List of Productions Eligible for the 88Th Academy Awards
REMINDER LIST OF PRODUCTIONS ELIGIBLE FOR THE 88TH ACADEMY AWARDS ADULT BEGINNERS Actors: Nick Kroll. Bobby Cannavale. Matthew Paddock. Caleb Paddock. Joel McHale. Jason Mantzoukas. Mike Birbiglia. Bobby Moynihan. Actresses: Rose Byrne. Jane Krakowski. AFTER WORDS Actors: Óscar Jaenada. Actresses: Marcia Gay Harden. Jenna Ortega. THE AGE OF ADALINE Actors: Michiel Huisman. Harrison Ford. Actresses: Blake Lively. Kathy Baker. Ellen Burstyn. ALLELUIA Actors: Laurent Lucas. Actresses: Lola Dueñas. ALOFT Actors: Cillian Murphy. Zen McGrath. Winta McGrath. Peter McRobbie. Ian Tracey. William Shimell. Andy Murray. Actresses: Jennifer Connelly. Mélanie Laurent. Oona Chaplin. ALOHA Actors: Bradley Cooper. Bill Murray. John Krasinski. Danny McBride. Alec Baldwin. Bill Camp. Actresses: Emma Stone. Rachel McAdams. ALTERED MINDS Actors: Judd Hirsch. Ryan O'Nan. C. S. Lee. Joseph Lyle Taylor. Actresses: Caroline Lagerfelt. Jaime Ray Newman. ALVIN AND THE CHIPMUNKS: THE ROAD CHIP Actors: Jason Lee. Tony Hale. Josh Green. Flula Borg. Eddie Steeples. Justin Long. Matthew Gray Gubler. Jesse McCartney. José D. Xuconoxtli, Jr.. Actresses: Kimberly Williams-Paisley. Bella Thorne. Uzo Aduba. Retta. Kaley Cuoco. Anna Faris. Christina Applegate. Jennifer Coolidge. Jesica Ahlberg. Denitra Isler. 88th Academy Awards Page 1 of 32 AMERICAN ULTRA Actors: Jesse Eisenberg. Topher Grace. Walton Goggins. John Leguizamo. Bill Pullman. Tony Hale. Actresses: Kristen Stewart. Connie Britton. AMY ANOMALISA Actors: Tom Noonan. David Thewlis. Actresses: Jennifer Jason Leigh. ANT-MAN Actors: Paul Rudd. Corey Stoll. Bobby Cannavale. Michael Peña. Tip "T.I." Harris. Anthony Mackie. Wood Harris. David Dastmalchian. Martin Donovan. Michael Douglas. Actresses: Evangeline Lilly. Judy Greer. Abby Ryder Fortson. Hayley Atwell. ARDOR Actors: Gael García Bernal. Claudio Tolcachir. -

Other 2:6:06 Pg 1.Pdf
Colby Free Press Monday, February 6, 2006 Page 5 Go Eagles! TV LISTINGS sponsored by the COLBY FREE PRESS WEEKDAYS FEBRUARY 7 - FEBRUARY 13 6 AM 6:30 7 AM 7:30 8 AM 8:30 9 AM 9:30 10 AM 10:30 11 AM 11:30 KLBY/ABC Good Morning Good Morning America Martha The View Million- News H h Kansas aire KSNK/NBC News Cont’d Today Live With Regis Paid Paid L j and Kelly Program Program KBSL/CBS News Cont’d News The Early Show The 700 Club The Price Is Right The Young and the 1< NX Restless K15CG BBC Body Caillou Mister Beren. Cliff Pup Big Big Dragon Sesame Street Teletub- Barney- d World Electric Rogers Bears World Tales bies Friends ESPN Sports- Varied SportsCenter SportsCenter Sports- Varied SportsCenter NFL Live Varied O_ Center Center USA Walker, Texas JAG The District Nash Bridges Nash Bridges Coach Varied P^ Ranger TBS Saved Saved Saved Saved Dawson’s Creek Movie Becker Becker P_ by Bell by Bell by Bell by Bell WGN Chang- Believer Happy Happy Hillbil- Hillbil- Rockford Files Matlock Magnum, P.I. Pa ing Voice Days Days lies lies TNT Angel Charmed Charmed ER ER Judging Amy QW DSC Paid Paid Paid Paid American Chopper American Chopper Varied Programs QY Program Program Program Program A&E Classroom Varied Programs City Confidential American Justice Q^ HIST Varied Programs Q_ NICK Oddpar- Oddpar- Spon- Spon- Dora the Go Blue’s Backyar- Dora the Lazy- Max & Dora the TISHA COX/Colby Free Press Rl ents ents geBob geBob Explorer Diego Clues digans Explorer Town Ruby Explorer DISN Break- Higgly- Ein- JoJo’s Doodle- Charlie The Koala Rolie Doodle- Charlie Rolie The Colby High School cheerleaders cheered on the varsity wrestling team during their dual R] fast Bear town steins Circus bops & Lola Wiggles Brothers Polie bops & Lola Polie against Oberlin Friday at the high school gym. -

2015 Annual Report
2015 ANNUAL REPORT VICTOR MATOS VAZQUEZ ADRIAN MATRAM JASON LEWIS MATSON MATTHEW MATSON SCOTT P MATSON MICHAEL A MATTEO SPENCER W MATTERS JASON P MATTHES JEFF ALLEN MATTHES JUSTIN MATTHES SUPPLE MATTHEW BILLY GENE MATTHEWS JR BLAIN MATTHEWS BOBBY N MATTHEWS BRIAN K MATTHEWS CHRISTOPHER SCOTT MATTHEWS DAMON A MATTHEWS DAVID MATTHEWS DENNIS MATTHEWS EUGENE MATTHEWS JAMES B MATTHEWS JONATHAN H MATTHEWS KARL MATTHEWS KENNETH E MATTHEWS MARK R MATTHEWS MARY C MATTHEWS MICHAEL D MATTHEWS MICHAEL E MATTHEWS PAUL MATTHEWS RAY E MATTHEWS JR RHONDA MATTHEWS ROBERT A MATTHEWS RYAN S MATTHEWS STEPHANIE R MATTHEWS TERESA A MATTHEWS TERRY MATTHEWS TRACY MATTHEWS MITCHELL D MATTINGLY KYLE MATTINSON JOHN MATTOCKS JEFFERY A MATTOX JEFFERY ALLEN MATTOX II JOSHUA JDM MATTSON KAM MATTU JOSEPH J MATUSKA TAMMY MATUSZAK MANUEL MATUTE BACHES JOSEPH MAUGERI BRUCE MAUGHAN GAYLEN C MAUGHAN JAYDEN MAUGHAN JORDAN R MAUGHAN THOMAS J MAUK ANDREW FERRELL MAULDIN ARTHUR L MAULDIN CHELSI MAULDIN MICHAEL MAULDIN KYLIE MAUNDRELL RANDY K MAURER TOBIAS MAURICE MARIBEL MAURICIO ANGELINE MAURO DAVID L MAUSEHUND KEVIN A MAVIS ANTHONY D MAXWELL CAMERON MAXWELL COLTON D MAXWELL DAVIS MAXWELL ERIC L MAXWELL JAMES TONY MAXWELL KIM MAXWELL MICHAEL W MAXWELL RODNEY MAXWELL CRAIG C MAY CRAIG M MAY GORDON D MAY JAMES A MAY JEDIDIAH E MAY JEREMY M MAY JOSEPH CHRISTOPHER MAY JUSTIN MAY KELVIN MAY KENNETH D MAY MARK E MAY MICHEAL T MAY SCOTT A MAY WILLIE D MAY DANIEL MAYA DONALD GENE MAYBERRY MARLON C MAYBERRY CLAYTON TYLER MAYER JOHN MAYER JOHN ZACHARY MAYER TROY MAYER JULIAN L MAYERS -

Network Telivision
NETWORK TELIVISION NETWORK SHOWS: ABC AMERICAN HOUSEWIFE Comedy ABC Studios Kapital Entertainment Wednesdays, 9:30 - 10:00 p.m., returns Sept. 27 Cast: Katy Mixon as Katie Otto, Meg Donnelly as Taylor Otto, Diedrich Bader as Jeff Otto, Ali Wong as Doris, Julia Butters as Anna-Kat Otto, Daniel DiMaggio as Harrison Otto, Carly Hughes as Angela Executive producers: Sarah Dunn, Aaron Kaplan, Rick Weiner, Kenny Schwartz, Ruben Fleischer Casting: Brett Greenstein, Collin Daniel, Greenstein/Daniel Casting, 1030 Cole Ave., Los Angeles, CA 90038 Shoots in Los Angeles. BLACK-ISH Comedy ABC Studios Tuesdays, 9:00 - 9:30 p.m., returns Oct. 3 Cast: Anthony Anderson as Andre “Dre” Johnson, Tracee Ellis Ross as Rainbow Johnson, Yara Shahidi as Zoey Johnson, Marcus Scribner as Andre Johnson, Jr., Miles Brown as Jack Johnson, Marsai Martin as Diane Johnson, Laurence Fishburne as Pops, and Peter Mackenzie as Mr. Stevens Executive producers: Kenya Barris, Stacy Traub, Anthony Anderson, Laurence Fishburne, Helen Sugland, E. Brian Dobbins, Corey Nickerson Casting: Alexis Frank Koczaraand Christine Smith Shevchenko, Koczara/Shevchenko Casting, Disney Lot, 500 S. Buena Vista St., Shorts Bldg. 147, Burbank, CA 91521 Shoots in Los Angeles DESIGNATED SURVIVOR Drama ABC Studios The Mark Gordon Company Wednesdays, 10:00 - 11:00 p.m., returns Sept. 27 Cast: Kiefer Sutherland as Tom Kirkman, Natascha McElhone as Alex Kirkman, Adan Canto as Aaron Shore, Italia Ricci as Emily Rhodes, LaMonica Garrett as Mike Ritter, Kal Pennas Seth Wright, Maggie Q as Hannah Wells, Zoe McLellan as Kendra Daynes, Ben Lawson as Damian Rennett, and Paulo Costanzo as Lyor Boone Executive producers: David Guggenheim, Simon Kinberg, Mark Gordon, Keith Eisner, Jeff Melvoin, Nick Pepper, Suzan Bymel, Aditya Sood, Kiefer Sutherland Casting: Liz Dean, Ulrich/Dawson/Kritzer Casting, 4705 Laurel Canyon Blvd., Ste. -

STEVEN KRIMMEL [email protected] | (510) 996-2292
SK STEVEN KRIMMEL [email protected] | (510) 996-2292 www.stevenkrimmel.com | https://vimeo.com/275176868 PROFESSIONAL I am a passionate, hard-working, team player who loves both the art and technical SUMMARY sides of editing. My experience includes working as an assistant editor in professional television settings and am frequently freelancing as the main editor on narrative projects. I desire post-production positions that allow me to enhance my knowledge and skill set, but am ultimately seeking to support and elevate quality media and stories. SKILLS Proficiency in: Adobe Premiere, Communicates effectively with Adobe After Effects, Adobe Media team Encoder, Final Cut Pro 7 Takes ownership of Experience: Adobe Creative Suite, post-production decision making Avid Media Composer, Avid Pro Well-versed in current Film/TV Tools, Microsoft Office, Final and Film History Draft, Highland, DIT, Narrative Film Directing, Production Sound Recording Keeps workspace organized WORK HISTORY Editor | KrimStine Productions - Los Angeles, CA 2013 - CURRENT - Editor of 2019 short film "Concealer" in collaboration with Amazon Fire TV and ATTN: - Editor of "Starring Kristine" shorts featured in the Toronto International Film Festival's TIFFxInstagram Shorts Festival 2018 Assistant Editor | NuvoTV (merged With Fuse In 2015) - 2014 - 2014 Glendale, CA - NUVOtv's "Nu Point of View: The Emerging Latino Filmmakers" Assistant Editor | OASIS TV - Century City, CA 2011 - 2011 Assistant Editor | Indigo Films - San Rafael, CA 2010 - 2011 - Investigation Discovery -
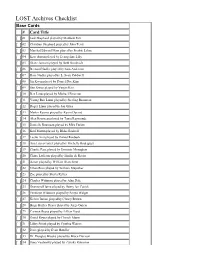
LOST Archives Checklist
LOST Archives Checklist Base Cards # Card Title [ ] 01 Jack Shephard played by Matthew Fox [ ] 02 Christian Shephard played by John Terry [ ] 03 Marshal Edward Mars played by Fredric Lehne [ ] 04 Kate Austin played by Evangeline Lilly [ ] 05 Diane Janssen played by Beth Broderick [ ] 06 Bernard Nadler played by Sam Anderson [ ] 07 Rose Nadler played by L. Scott Caldwell [ ] 08 Jin Kwon played by Daniel Dae Kim [ ] 09 Sun Kwon played by Yunjin Kim [ ] 10 Ben Linus played by Michael Emerson [ ] 11 Young Ben Linus played by Sterling Beaumon [ ] 12 Roger Linus played by Jon Gries [ ] 13 Martin Keamy played by Kevin Durand [ ] 14 Alex Rousseau played by Tania Raymonde [ ] 15 Danielle Rousseau played by Mira Furlan [ ] 16 Karl Martin played by Blake Bashoff [ ] 17 Leslie Arzt played by Daniel Roebuck [ ] 18 Ana Lucia Cortez played by Michelle Rodriguez [ ] 19 Charlie Pace played by Dominic Monaghan [ ] 20 Claire Littleton played by Emilie de Ravin [ ] 21 Aaron played by William Blanchette [ ] 22 Ethan Rom played by William Mapother [ ] 23 Zoe played by Sheila Kelley [ ] 24 Charles Widmore played by Alan Dale [ ] 25 Desmond Hume played by Henry Ian Cusick [ ] 26 Penelope Widmore played by Sonya Walger [ ] 27 Kelvin Inman played by Clancy Brown [ ] 28 Hugo Hurley Reyes played by Jorge Garcia [ ] 29 Carmen Reyes played by Lillian Hurst [ ] 30 David Reyes played by Cheech Marin [ ] 31 Libby Smith played by Cynthia Watros [ ] 32 Dave played by Evan Handler [ ] 33 Dr. Douglas Brooks played by Bruce Davison [ ] 34 Ilana Verdansky played by Zuleika -

Enhancing TV Programmes with Additional Contents Using MPEG-7 Segmentation Information Q
Expert Systems with Applications 37 (2010) 1124–1133 Contents lists available at ScienceDirect Expert Systems with Applications journal homepage: www.elsevier.com/locate/eswa Enhancing TV programmes with additional contents using MPEG-7 segmentation information q Marta Rey-López *, Ana Fernández-Vilas, Rebeca P. Díaz-Redondo, Martín López-Nores, José J. Pazos-Arias, Alberto Gil-Solla, Manuel Ramos-Cabrer, Jorge García-Duque University of Vigo, Department of Telematics Engineering, 36310 Vigo, Spain article info abstract Keywords: Interactive Digital TV offers a large amount of TV channels, as well as new contents that come along with Metadata the TV programmes. To take advantage of these additional contents and make them easily available to MPEG-7 viewers, this paper proposes to offer additional contents linked to the segments of TV programmes by Video tagging means of semantic relations obtained using MPEG-7 segmentation information. As a practical use of this work, we propose two different application fields: t-learning, with the aim of using TV programmes to engage viewers in education; and personalised advertising, whose goal is offering viewers products of their interest, maximising its effectiveness. Ó 2009 Elsevier Ltd. All rights reserved. 1. Introduction these strategies, the programmes can be grouped together accord- ing to two different criteria: the similarity in their contents (con- The arrival of Interactive Digital TV (IDTV) permits viewers to tent-based filtering) and the resemblance between the profiles of access a huge amount of interactive contents, in addition to the tra- the viewers that have watched them (collaborative filtering). In ditional TV programmes: games, web pages, learning contents, this manner, we could be able to offer related contents when the new types of advertisements, etc. -

SIPA's CLASS of 2021
CELEBRATING SIPA’s CLASS OF 2021 – VIRTUALLY Madeleine Albright and Eric Holder will address SIPA’s Class of 2021. 2 CONTENTS Graduation Address � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 4 – 5 Order of Exercises � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 6 Faculty of International and Public Affairs � � � � � � � � � � � � � � � � � � � � � � � � � � � 8 SIPA Faculty � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 8 Senior Research Scholars/Research Scholars � � � � � � � � � � � � � � � � � � � � � � � � � � � 8 SIPA’s Class of 2021 � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 9 PhD Graduates � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 9 MPA Graduates � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 9 – 13 EMPA Graduates � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 13 – 14 MPA-DP Graduates � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 14 – 15 MPA-EPM Graduates � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 15 MPA-ESP Graduates � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � 15 – 16 MIA Graduates � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � � �