Gastropoda: Pulmonata: Planorbidae) on Different Types of Substratum In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beiträge Zur Kenntnis Der Paläarktischen Planorbidae. Wilhelm A. Lindholm
Heft 6 Jahrgang LVIII. 1926 Archiv ihr Molluskenkonde Beiträge zur Kenntnis der paläarktischen Planorbidae. Von W. A. Lindholm. I. Zur Synonymie des Planorbis crista (L.). Es ist eine bekannte Tatsache, daß unser kleinster Planorbis hinsichtlich der Skulptur seines Gehäuses verschiedene Varietäten darstellt, welche Eigenschaft ihm im Laufe der Zeiten eine stattliche Anzahl von Artnamen eingebracht hat. Es hatte lange gedauert, bis sich die Forscher überzeugt hatten, daß es sich nicht um mehrere Arten, sondern nur um Formen und z. T. sogar um Wachstumserscheinungen einer einzigen Art handelt. Es kommen eigentlich' nur zwei Formen in Betracht: eine glatte d. h. nur feingestreifte, nicht quer- gerippte, welche meist von den Autoren bis auf den heutigen Tag als PL nautileus (L.) und eine mit ian der Peripherie höckerartig vortretenden Querrippen versehene, welche als PL crista (L.) oder PL cristatus D rap , aufgeführt wird, und deren Jugendtracht an der Peripherie des letzten Umgangs mehr weniger lange Stacheln auf weist. Diese Jugendtracht bewahren ein zelne Individuen auch während ihrer weiteren Lebens dauer; solche Stücke sind von S. CI es sin als PL crista var. spinulosus in die Wissenschaft eingeführt worden. 242 Nun beruht diese landläufige Nomenklatur auf einen Irrtum, da Linné beide erwähnte Artnamen1) auf dasselbe Tier oder richtiger auf dieselbe Abbildung bei Rösel* 2) begründet hatte. Diese Figur stellt nun diejenige Form dar, welche dessin als var. spinulosus bezeichnet hatte, was schon aus RöseFs Worten her vorgeht: ,,Dieses Ammonshorn ist nicht nur gleichsam mit Reifen umleget, sondern es hat auch an seinem Rucken auf jedem Reif eines Stachelspitze.“ Die durch Linné vorgenommene doppelte Benennung dieser Art war seinen Zeitgenossen nicht entgangen, ist aber in unseren Tagen anscheinend vergessen worden. -

Gyraulus Laevis in Nederland 87
Kuijper: Gyraulus laevis in Nederland 87 Gyraulus laevis (Mollusca: Planorbidae) in Nederland door W.J. Kuijper Enkele recente waarnemingen van een van onze zeldzaamste planorbiden waren de aanleiding tot het samenstellen van een over- laevis zicht van alle van Nederland bekende vondsten van Gyraulus (Alder, 1838). Tot voor kort waren slechts enkele vindplaatsen van dit dier bekend (Janssen & De Vogel, 1965: 75). Hierbij komt het betrof feit dat het in een aantal gevallen slechts een enkel exemplaar en de soort niet meer teruggevonden kon worden. Het volgende geeft een chronologisch overzicht van de waarnemingen van Gyraulus laevis in Nederland. Voor zover beschikbaar zijn diverse gegevens van de vindplaatsen vermeld. WAARNEMINGEN 1. Koudekerke (bij Middelburg), buitenplaats Vijvervreugd, ± 1890. Fraai vers materiaal: 69 exemplaren in de collectie Schep- de man (Zoölogisch Museum, Amsterdam). Later niet meer vermeld, buitenplaats bestaat niet meer (Kuiper, 1944: 6). Dit materiaalwerd verzameld door Dr. IJ. Keijzer en is vermeld in: Verslag over 1885-1893 van het Zeeuwsch Genootschap der Wetenschappen, blz. 88 (volgens Mevr. Dr. W.S.S. van der Feen - van Benthem Jut- ting). Verder is materiaal van deze vindplaats in de collecties van het Zeeuwsch Museum te Middelburg (2 ex.) en het Natuurhistorisch Museum te Enschede (6 ex.) aanwezig. 2. Warmond (bij Leiden), in de Leede, januari 1916 (Van Ben- them Jutting, 1933: 179). Dit materiaal werd verzameld door P.P. de Koning; in het Rijksmuseum van Natuurlijke Historie te Leiden bevinden zich vier schelpen van deze vindplaats, waarvan twee een doorsnede van ca. 4 mm bereiken. 3. 't Zand (bij Roodeschool), Oostpolder, 1927 (Van Benthem Jutting, 1947: 66). -

WMSDB - Worldwide Mollusc Species Data Base
WMSDB - Worldwide Mollusc Species Data Base Family: TURBINIDAE Author: Claudio Galli - [email protected] (updated 07/set/2015) Class: GASTROPODA --- Clade: VETIGASTROPODA-TROCHOIDEA ------ Family: TURBINIDAE Rafinesque, 1815 (Sea) - Alphabetic order - when first name is in bold the species has images Taxa=681, Genus=26, Subgenus=17, Species=203, Subspecies=23, Synonyms=411, Images=168 abyssorum , Bolma henica abyssorum M.M. Schepman, 1908 aculeata , Guildfordia aculeata S. Kosuge, 1979 aculeatus , Turbo aculeatus T. Allan, 1818 - syn of: Epitonium muricatum (A. Risso, 1826) acutangulus, Turbo acutangulus C. Linnaeus, 1758 acutus , Turbo acutus E. Donovan, 1804 - syn of: Turbonilla acuta (E. Donovan, 1804) aegyptius , Turbo aegyptius J.F. Gmelin, 1791 - syn of: Rubritrochus declivis (P. Forsskål in C. Niebuhr, 1775) aereus , Turbo aereus J. Adams, 1797 - syn of: Rissoa parva (E.M. Da Costa, 1778) aethiops , Turbo aethiops J.F. Gmelin, 1791 - syn of: Diloma aethiops (J.F. Gmelin, 1791) agonistes , Turbo agonistes W.H. Dall & W.H. Ochsner, 1928 - syn of: Turbo scitulus (W.H. Dall, 1919) albidus , Turbo albidus F. Kanmacher, 1798 - syn of: Graphis albida (F. Kanmacher, 1798) albocinctus , Turbo albocinctus J.H.F. Link, 1807 - syn of: Littorina saxatilis (A.G. Olivi, 1792) albofasciatus , Turbo albofasciatus L. Bozzetti, 1994 albofasciatus , Marmarostoma albofasciatus L. Bozzetti, 1994 - syn of: Turbo albofasciatus L. Bozzetti, 1994 albulus , Turbo albulus O. Fabricius, 1780 - syn of: Menestho albula (O. Fabricius, 1780) albus , Turbo albus J. Adams, 1797 - syn of: Rissoa parva (E.M. Da Costa, 1778) albus, Turbo albus T. Pennant, 1777 amabilis , Turbo amabilis H. Ozaki, 1954 - syn of: Bolma guttata (A. Adams, 1863) americanum , Lithopoma americanum (J.F. -

Planorbidae) from New Mexico
FRONT COVER—See Fig. 2B, p. 7. Circular 194 New Mexico Bureau of Mines & Mineral Resources A DIVISION OF NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY Pecosorbis, a new genus of fresh-water snails (Planorbidae) from New Mexico Dwight W. Taylor 98 Main St., #308, Tiburon, California 94920 SOCORRO 1985 iii Contents ABSTRACT 5 INTRODUCTION 5 MATERIALS AND METHODS 5 DESCRIPTION OF PECOSORBIS 5 PECOSORBIS. NEW GENUS 5 PECOSORBIS KANSASENSIS (Berry) 6 LOCALITIES AND MATERIAL EXAMINED 9 Habitat 12 CLASSIFICATION AND RELATIONSHIPS 12 DESCRIPTION OF MENETUS 14 GENUS MENETUS H. AND A. ADAMS 14 DESCRIPTION OF MENETUS CALLIOGLYPTUS 14 REFERENCES 17 Figures 1—Pecosorbis kansasensis, shell 6 2—Pecosorbis kansasensis, shell removed 7 3—Pecosorbis kansasensis, penial complex 8 4—Pecosorbis kansasensis, reproductive system 8 5—Pecosorbis kansasensis, penial complex 9 6—Pecosorbis kansasensis, ovotestis and seminal vesicle 10 7—Pecosorbis kansasensis, prostate 10 8—Pecosorbis kansasensis, penial complex 10 9—Pecosorbis kansaensis, composite diagram of penial complex 10 10—Pecosorbis kansasensis, distribution map 11 11—Menetus callioglyptus, reproductive system 15 12—Menetus callioglyptus, penial complex 15 13—Menetus callioglyptus, penial complex 16 14—Planorbella trivolvis lenta, reproductive system 16 Tables 1—Comparison of Menetus and Pecosorbis 13 5 Abstract Pecosorbis, new genus of Planorbidae, subfamily Planorbulinae, is established for Biomphalaria kansasensis Berry. The species has previously been known only as a Pliocene fossil, but now is recognized in the Quaternary of the southwest United States, and living in the Pecos Valley of New Mexico. Pecosorbis is unusual because of its restricted distribution and habitat in seasonal rock pools. Most similar to Menetus, it differs in having a preputial organ with an external duct, no spermatheca, and a penial sac that is mostly eversible. -

Malacostratigraphical Investigation of the Late Quaternary Subsided Zones of Hungary
Fol. Hist.-nat. Mus. Matr., 17: 97-106, 1992 Malacostratigraphical Investigation of the Late Quaternary Subsided Zones of Hungary FŰKÖH Levente ABSTRACT: The author briefly performs the ecelogical, biostratigraphical and malakostratigraphical elaboration of the Mollusc faunae of the most complete sequences and with the knowledge of the results gives the general concludes. During the investigation of the Hungarian Holocene Mollusc-fauna more and more and Important flat-land fauna became known, beside the faunae of the medium high mountain-ranges. The increase of these data enabled to attempt with full knowledge of the facts until now - giving outline history of the succession of the juvenile subsided zones with the help of malacostratigraphy. Because the ecological conditions of the species of our fresh-water fauna is less explored than the ecological conditions of the terrestrial ones,so such detailed analysis cannot be expected than have been made from the medium high mountain territories (FUKÜH, L. 1987). The faunal evolution of these territories (Fig. 1.) is approximately the same, it is why we made attempt to publish the fundamental Informations about courses of development and stratigraphical examinations. In the next part of this paper I Introduce to biostratigraphical-malacostratigraphical elaboration of the most complete sequences and with the knowledge of the results I give the general concludes. MALACOSTRATIGRAPHICAL INVESTIGATION OF THE TRANSDANUBIA 5árrét, Fejér county The finding place can be found at the Transdanubian part of Hungary at the so celled Mezőföld (Ä0ÄM, L. - MAROSI, S. - SZILÄRD, J. 1955). The first collection, with the consideration of stratigraphical data was done by E. -

On the Presence of the Alien Freshwater Gastropod Ferrissia Fragilis (Tryon, 1863) (Gastropoda: Planorbidae) in the Maltese Islands (Central Mediterranean)
Boll. Malacol., 45: 123-127 (2/2009) On the presence of the alien freshwater gastropod Ferrissia fragilis (Tryon, 1863) (Gastropoda: Planorbidae) in the Maltese Islands (Central Mediterranean) David P. Cilia 29, Red Palace Way, Abstract Santa Venera SVR1454, An established population of the North-American freshwater gastropod Ferrissia fragilis (Tryon, 1863) is Malta, recorded from the island of Malta (Central Mediterranean) for the first time. This population was found in [email protected] an anthropogenic habitat at the northeast of Malta. Ferrissia fragilis is an invader of several freshwater habitats throughout Europe and beyond. If released into the wild, it could present competition for threat- ened Maltese freshwater Mollusca. Riassunto Una popolazione stabile del gasteropode d’acqua dolce, di origine nord americana, Ferrissia fragilis (Tryon, 1863) è segnalata per la prima volta a Malta (Mediterraneo centrale). La popolazione è stata trovata in un ambiente antropizzato, nella parte nord-orientale di Malta. Ferrissia fragilis è un invasore di diversi ambien- ti d’acqua dolce in Europa ed altre aree. Se rilasciato negli ambienti naturali, questa specie potrebbe en- trare in competizione con le specie autoctone e minacciare la fauna dulcicola di Malta. Key words Gastropoda, Planorbidae, Ferrissia fragilis, freshwater, alien species, Malta. Introduction tion and habitat were collected and also preserved in 90% alcohol for further investigation. The alien non-marine gastropods of the Maltese Islands have been studied in detail by various authors (Tab. 1). Material studied: Blata l-Bajda, Malta; 18.III.2009, 28. Giusti et al. (1995) list eight species as being of non-na- IV.2009, 12.V.2009 & 1.VI.2009, several live individuals tive or reintroduced origin, of which two are restricted in situ; David P. -
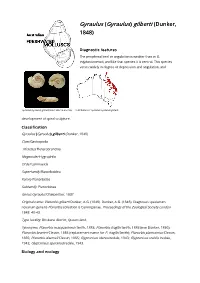
Gyraulus) Gilberti (Dunker, 1848
Gyraulus (Gyraulus) gilberti (Dunker, 1848) Diagnostic features The peripheral keel or angulation is weaker than in G. edgbastonensis, and like that species it is central. This species varies widely in degree of depression and angulation, and Gyraulus (Gyraulus) gilberti (adult size 4.8-5.5 mm) Distribution of Gyraulus (Gyraulus) gilberti. development of spiral sculpture. Classification Gyraulus (Gyraulus) gilberti (Dunker, 1848) Class Gastropoda I nfraclass Heterobranchia Megaorder Hygrophila Order Lymnaeida Superfamily Planorboidea Family Planorbidae Subfamily: Planorbinae Genus Gyraulus Charpentier, 1837 Original name: Planorbis gilberti Dunker, A.G. (1848). Dunker, A.G. (1848). Diagnoses specierum novarum generis Planorbis collection is Cumingianae. Proceedings of the Zoological Society London 1848: 40-43. Type locality: Brisbane district, Queensland. Synonyms: Planorbis macquariensis Smith, 1883; Planorbis fragilis Smith, 1883 (non Dunker, 1850); Planorbis brazieri Clessin, 1885 (replacement name for P. fragilis Smith); Planorbis planissimus Clessin, 1885; Planorbis daemeli Clessin, 1885; Glyptanisus idenusredale, 1943; Glyptanisus stabilis redale, 1943; Glyptanisus speranusredale, 1943. Biology and ecology This species lives in water weeds and other vegetation in ponds, billabongs, swamps and sluggish streams and rivers in tropical and subtropical eastern Australia. Feeds on detritus. Egg mass presumably a jelly strip containing small eggs. Development direct. Brown (2001) described the anatomy of this species. This species is an intermediate host for the stomach fluke Orthocoelium streptocoelium (Boray, 1982; Beesley et al., 1998). Distribution This species occurs throughout eastern Australia, from Cape York to northern New South Wales. Notes G. isingi and/or G.waterhousei may possibly be conspecific with this species (Brown, 2001). Further reading Beesley, P. L., Ross, G. J. -

Anisus Vorticulus (Troschel 1834) (Gastropoda: Planorbidae) in Northeast Germany
JOURNAL OF CONCHOLOGY (2013), VOL.41, NO.3 389 SOME ECOLOGICAL PECULIARITIES OF ANISUS VORTICULUS (TROSCHEL 1834) (GASTROPODA: PLANORBIDAE) IN NORTHEAST GERMANY MICHAEL L. ZETTLER Leibniz Institute for Baltic Sea Research Warnemünde, Seestr. 15, D-18119 Rostock, Germany Abstract During the EU Habitats Directive monitoring between 2008 and 2010 the ecological requirements of the gastropod species Anisus vorticulus (Troschel 1834) were investigated in 24 different waterbodies of northeast Germany. 117 sampling units were analyzed quantitatively. 45 of these units contained living individuals of the target species in abundances between 4 and 616 individuals m-2. More than 25.300 living individuals of accompanying freshwater mollusc species and about 9.400 empty shells were counted and determined to the species level. Altogether 47 species were identified. The benefit of enhanced knowledge on the ecological requirements was gained due to the wide range and high number of sampled habitats with both obviously convenient and inconvenient living conditions for A. vorticulus. In northeast Germany the amphibian zones of sheltered mesotrophic lake shores, swampy (lime) fens and peat holes which are sun exposed and have populations of any Chara species belong to the optimal, continuously and densely colonized biotopes. The cluster analysis emphasized that A. vorticulus was associated with a typical species composition, which can be named as “Anisus-vorticulus-community”. In compliance with that both the frequency of combined occurrence of species and their similarity in relative abundance are important. The following species belong to the “Anisus-vorticulus-community” in northeast Germany: Pisidium obtusale, Pisidium milium, Pisidium pseudosphaerium, Bithynia leachii, Stagnicola palustris, Valvata cristata, Bathyomphalus contortus, Bithynia tentaculata, Anisus vortex, Hippeutis complanatus, Gyraulus crista, Physa fontinalis, Segmentina nitida and Anisus vorticulus. -

Zierliche Tellerschnecke (Anisus Vorticulus)
HESSEN-FORST Artensteckbrief Zierliche Tellerschnecke (Anisus vorticulus) Stand: 2006 weitere Informationen erhalten Sie bei: Hessen-Forst FENA Naturschutz Europastraße 10 - 12 35394 Gießen Tel.: 0641 / 4991-264 E-Mail: [email protected] Art Deutscher Name: Zierliche Tellerschnecke, Anisus (Disculifer ) vorticulus (T ROSCHEL 1834) Synonyme: Planorbis vorticulus, Spiralina vorticulus, Planorbis acies, Gyrorbis vorticulus, Planorbis charteus, Planorbis bavarica, Gyrorbis helveticus Systematische Einordnung Reich: Mollusca CUVIER 1795 Klasse: Gastropoda CUVIER 1795 Unterklasse: Orthogastropoda PELSENEER 1889 Überordnung: Heterobranchia J. E. GRAY 1840 Ordnung: Pulmonata CUVIER in BLAINVILLE 1814 Unterordnung: Basommatophora KEFERSTEIN 1864 Überfamilie: Planorboidea RAFINISQUE 1815 Familie: Planorbidae RAFINISQUE 1815 Unterfamilie: Planorbinae RAFINISQUE 1815 Gattung: Anisus S. STUDER 1820 Untergattung: Disculifer C. BOETTGER 1944 Verbreitung und Bestandsentwicklung Gesamt-Verbreitung: Die Gesamtart besiedelt Ost- und Mittel-Europa, die Britischen Inseln nur in Teilen (Sussex, Norfolk). Sie reicht im Süden bis ins Burgenland, nach Nord-Tirol, Vorarlberg und die Schweiz (Glöer 2002), im Westen mit nur wenigen verstreute Fundorten in Frankreich (Thonon, Rhone-Becken, Ried) (FALKNER & al. 2002), keine in Belgien, zahlreiche in den Niederlanden, vereinzelte in Süd-Dänemark (GLÖER 2002). Regionale Verbreitung: In Hessen ist Anisus vorticulus von einem einzigen Fundort bei Trebur (Hessisches Ried) bekannt (PETRY 1925). Dieses Vorkommen -

Freshwater Snail Guide
A Beginner’s Guide to Freshwater Snails of Central Scotland Freshwater snails are a diverse and fascinating group of molluscs found in ponds, lochs, canals, rivers and watery ditches all across the globe. This guide is a simple introduction to some of the most commonly encountered and easily identifiable freshwater snail species in Central Scotland. How do I look for freshwater snails? 1) Choose your spot: anywhere with water – your garden pond, a nearby river, canal or loch – is likely to have snails. 2) Grab a pond net (buy online or make your own by attaching a sieve to the end of an old broom or mop), a white tray or tub and a hand-lens or magnifier. 3) Fill the tray with water from your chosen habitat. Sweep the net around in the water, making sure to get among any vegetation (don’t go too near the bottom or you’ll get a net full of mud), empty your net into the water-filled tray and identify what you have found! Snail shell features Spire Whorls Some snail species have a hard plate called an ‘operculum’ Height which they Mouth (Aperture) use to seal the mouth of the shell when they are inside Height Pointed shell Flat shell Width Width Pond Snails (Lymnaeidae) Variable in size. Mouth always on right-hand side, shells usually long and pointed. Great Pond Snail Common Pond Snail Lymnaea stagnalis Radix balthica Largest pond snail. Common in ponds Fairly rounded and ’fat’. Common in weedy lakes, canals and sometimes slow river still waters. pools. -

Pomacea Canaliculata) Behaviors in Different Water Temperature Gradients
water Article Comparison of Invasive Apple Snail (Pomacea canaliculata) Behaviors in Different Water Temperature Gradients Mi-Jung Bae 1, Eui-Jin Kim 1 and Young-Seuk Park 2,* 1 Nakdonggang National Institute of Biological Resources, Sangju 37242, Korea; [email protected] (M.-J.B.); [email protected] (E.-J.K.) 2 Department of Biology, Kyung Hee University, Dongdaemun, Seoul 02447, Korea * Correspondence: [email protected]; Tel.: +82-2-961-0946 Abstract: Pomacea canaliculata (known as invasive apple snail) is a freshwater snail native to South America that was introduced into many countries (including Asia and North America) as a food source or for organic farming systems. However, it has invaded freshwater ecosystems and become a serious agricultural pest in paddy fields. Water temperature is an important factor determining behavior and successful establishment in new areas. We examined the behavioral responses of P. canaliculata with water temperature changes from 25 ◦C to 30 ◦C, 20 ◦C, and 15 ◦C by quantifying changes in nine behaviors. At the acclimated temperature (25 ◦C), the mobility of P. canaliculata was low during the day, but high at night. Clinging behavior increased as the water temperature decreased from 25 ◦C to 20 ◦C or 15 ◦C. Conversely, ventilation and food consumption increased when the water temperature increased from 25 ◦C to 30 ◦C. A self-organizing map (an unsupervised artificial neural network) was used to classify the behavioral patterns into seven clusters at different water temperatures. These results suggest that the activity levels or certain behaviors of P. canaliculata vary with the water temperature conditions. -

Aplexa Hypnorum (Gastropoda: Physidae) Exerts Competition on Two Lymnaeid Species in Periodically Dried Ditches
Ann. Limnol. - Int. J. Lim. 52 (2016) 379–386 Available online at: Ó The authors, 2016 www.limnology-journal.org DOI: 10.1051/limn/2016022 Aplexa hypnorum (Gastropoda: Physidae) exerts competition on two lymnaeid species in periodically dried ditches Daniel Rondelaud, Philippe Vignoles and Gilles Dreyfuss* Laboratory of Parasitology, Faculty of Pharmacy, 87025 Limoges Cedex, France Received 26 November 2014; Accepted 2 September 2016 Abstract – Samples of adult Aplexa hypnorum were experimentally introduced into periodically dried ditches colonized by Galba truncatula or Omphiscola glabra to monitor the distribution and density of these snail species from 2002 to 2008, and to compare these values with those noted in control sites only frequented by either lymnaeid. The introduction of A. hypnorum into each ditch was followed by the progressive coloni- zation of the entire habitat by the physid and progressive reduction of the portion occupied by the lymnaeid towards the upstream extremity of the ditch. Moreover, the size of the lymnaeid population decreased significantly over the 7-year period, with values noted in 2008 that were significantly lower than those recorded in 2002. In contrast, the mean densities were relatively stable in the sites only occupied by G. truncatula or O. glabra. Laboratory investigations were also carried out by placing juvenile, intermediate or adult physids in aquaria in the presence of juvenile, intermediate or adult G. truncatula (or O. glabra) for 30 days. The life stage of A. hypnorum had a significant influence on the survival of each lymnaeid. In snail combinations, this survival was significantly lower for adult G. truncatula (or O.