Differentiation of the Can F 1 Allergen in Hypoallergenic Dog Saliva Compared to Shedding Dog Saliva
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

British Veterinary Association / Kennel Club Hip Dysplasia Scheme
British Veterinary Association / Kennel Club Hip Dysplasia Scheme Breed Specific Statistics – 1 January 2001 to 31 December 2016 Hip scores should be considered along with other criteria as part of a responsible breeding programme, and it is recommended that breeders choose breeding stock with hip scores around and ideally below the breed median score, depending on the level of HD in the breed. HD status of parents, siblings and progeny for Kennel Club registered dogs should also be considered, and these together with a three generation Health Test Pedigree may be downloaded via the Health Test Results Finder, available on the Kennel Club’s online health tool Mate Select (www.mateselect.org.uk). In addition, estimated breeding values (EBVs) are available for breeds in which a significant number of dogs have been graded, via the same link. For further advice on the interpretation and use of hip scores see www.bva.co.uk/chs The breed median score is the score of the ‘average’ dog in that breed (i.e. an equal number of dogs in that breed have better and worse scores). No. 15 year No. 15 year 5 year 5 year Breed score in Breed score in Range Median Median Range Median Median 15 years 15 years Affenpinscher 40 8 – 90 13 14 Beagle 62 8 - 71 16 17 Afghan Hound 18 0 – 73 8.5 27 Bearded Collie 1511 0 – 70 9 9 Airedale Terrier 933 4 – 72 11 10 Beauceron 42 2 – 23 10 10 Akita 1029 0 – 91 7 7 Belgian Shepherd 249 0 – 37 8 8 Dog (Groenendael) Alaskan Malamute 1248 0 – 78 10 10 Belgian Shepherd 16 5 - 16 10 14 Dog (Laekenois) Anatolian 63 3 – 67 9 -

Seward County Kennel Club
VOLUME 2018, ISSUE 9 OCTOBER 9, 2018 Seward County Kennel Club A CLUB FOR PERSONS INTERESTED IN DOGS AND THE SPORT OF DOGS. OFFICERS President A DESIGNER DOG-MAKER Tabitha Dvorak (402) 641-2614 [email protected] REGRETS HIS CREATION Vice President The inventor of the Labradoodle believe he created Cindy Hill (206) 715-9995 a Frankenstein. [email protected] I was in the lobby of a hotel in Toronto waiting to Secretary Pat Schindler (402) 797-2114 be picked up and taken to the venue where I was [email protected] scheduled to give a talk. A well-dressed middle-aged woman was standing nearby with a sand-colored Treasurer Mary Bristol (402) 366-2659 curly-haired dog. As I bent down to give the dog [email protected] a friendly pat, she announced to me, “Molly is a purebred Labradoodle, just like the one that Jennifer BOARD MEMBERS Aniston has.” Morgan Ehlers (402) 366-2788 [email protected] It amazes me how intelligent people can refer to an Kathy Jackson (402) 742-0534 intentionally crossbred dog, such as the Labradoodle, [email protected] as “purebred.” The Labradoodle is a cross between the Labrador Retriever and the Poodle. Linda Soukup (402) 545-2186 It avoids the negative label of “mutt” or “mongrel” because it is a deliberate crossbreeding, [email protected] and those who market such pups have come to refer to them as “designer dogs,” a label Tanya Williams (402) 761-3598 designed to give them a hint of sophistication and elitism. There are many designer [email protected] dogs now available and the majority involve crossbreeding Poodles with other breeds. -

Complaint for Damages and 13 Jessica Loy, Brittany Swigart, Equitable Relief Brandon Swigart, Jane Doe, 14 1
Electronically FILED by Superior Court of California, County of Los Angeles on 12/16/2019 03:16 PM Sherri R. Carter, Executive Officer/Clerk of Court, by R. Perez,Deputy Clerk 19STCV45035 Assigned for all purposes to: Stanley Mosk Courthouse, Judicial Officer: Barbara Scheper 1 JOSEPH W. COTCHETT (SBN 36324) [email protected] GARY A. PRAGLIN (SBN 101256) [email protected] 2 NEDA L. LOTFI (SBN 290213) [email protected] 3 COTCHETT, PITRE & McCARTHY, LLP 2716 Ocean Park Blvd., Suite 3088 4 Santa Monica, California 90405 Telephone: (310) 392-2008 5 Facsimile: (310) 392-0111 KELSEY R. EBERLY (SBN 301025) 6 [email protected] ALEXANDRA J. MONSON (SBN 324794) [email protected] 7 ANIMAL LEGAL DEFENSE FUND 525 E. Cotati Avenue Cotati, California 94931 8 Telephone: (707) 795-2533 Facsimile: (707) 795-7280 9 Attorneys for Plaintiffs 10 IN THE SUPERIOR COURT OF THE STATE OF CALIFORNIA 11 IN AND FOR THE COUNTY OF LOS ANGELES Case No.: 12 COMPLAINT FOR DAMAGES AND 13 JESSICA LOY, BRITTANY SWIGART, EQUITABLE RELIEF BRANDON SWIGART, JANE DOE, 14 1. Violation of Cal. Bus. & Prof. Code § RAMTIN MEHRVIJEH, JULIA 17200 (Unfair Competition Law) 15 SUMMER EVANS, AUSTIN 2. Corporations Code § 10404 MATELSON, EMILY KOVACH, JANE (Declaratory and Injunctive Relief) 16 ROE, and CARU SOCIETY FOR THE 3. Violation of Cal. Bus. & Prof. Code § PREVENTION OF CRUELTY TO 17500 (False and Misleading 17 ANIMALS Advertising) 18 4. Violation of Cal. Com. Code § 2313 Plaintiffs, (Breach of Express Warranty) 19 5. Violation of Cal. Civ. Code § 1790 v. (Breach of Implied Warranty) 20 6. Violation of Cal. -

Laboratory Report
220 E. Rowan, Suite 220 Spokane, Washington 99207 www.pawprintgenetics.com (509) 483-5950 Laboratory Report Laboratory #: 65640 Call Name: Lila Order #: 28585 Registered Name: Euro Goldendoodle's Lila Ordered By: Genny Hamka Breed: Goldendoodle Ordered: Sept. 21, 2017 Sex: Female Received: Sept. 28, 2017 DOB: April 2017 Reported: Oct. 6, 2017 Registration #: - Results: Disease Gene Genotype Interpretation Degenerative Myelopathy SOD1 WT/WT Normal (clear) Ichthyosis (Golden Retriever Type) PNPLA1 WT/WT Normal (clear) Neonatal Encephalopathy with Seizures ATF2 WT/WT Normal (clear) Progressive Retinal Atrophy, Golden Retriever 1 SLC4A3 WT/WT Normal (clear) Progressive Retinal Atrophy, Golden Retriever 2 TTC8 WT/M Carrier Progressive Retinal Atrophy, Progressive Rod-Cone PRCD WT/WT Normal (clear) Degeneration Von Willebrand Disease I VWF WT/WT Normal (clear) WT, wild type (normal); M, mutant; Y, Y chromosome (male) Interpretation: Molecular genetic analysis was performed for seven specific mutations reported to be associated with disease in dogs. We identified two normal copies of the DNA sequences in six of the mutations tested. Thus, this dog is not at an increased risk for the diseases associated with these six mutations. However, we identified one normal copy and one mutant copy of the DNA sequences for TTC8. Thus, this dog is a carrier of Progressive Retinal Atrophy, Golden Retriever 2. Recommendations: Progressive Retinal Atrophy, Golden Retriever 2 is inherited in an autosomal recessive fashion. Based on this, and the fact that this dog showed a mutation in one copy of the TTC8 gene, this dog is a carrier of this disease. Although dogs that carry only one copy of this mutation will not be clinically affected, if bred with another carrier, the pairing could produce affected offspring. -
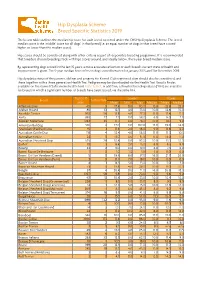
Hip Dysplasia Scheme Breed Specific Statistics 2019
Hip Dysplasia Scheme Breed Specific Statistics 2019 The below table outlines the median hip score for each breed screened under the CHS Hip Dysplasia Scheme. The breed median score is the ‘middle’ score for all dogs’ in that breed (i.e. an equal number of dogs in that breed have scored higher or lower than the median score). Hip scores should be considered along with other criteria as part of responsible breeding programme. It is recommended that breeders choose breeding stock with hips scores around, and ideally below, the 5-year breed median score. By representing dogs scored in the last 15 years, a more accurate reflection of each breed’s current state of health and improvement is given. The 5-year median here refers to dogs scored between 1st January 2015 and 31st December 2019. Hip dysplasia status of the parents, siblings and progeny for Kennel Club registered dogs should also be considered, and these together with a three generation Health Test Pedigree may be downloaded via the Health Test Results Finder, available on the Kennel Club's online health tool Mate Select. In addition, estimated breeding values (EBVs) are available for breeds in which a significant number of breeds have been scored, via the same link. Tested 15 15 years 5 years Breed Tested 2019 years Mean Min Max Median Mean Median Affenpinscher 40 0 17.9 8.0 90.0 13.0 23.8 23.0 Afghan Hound 85 33 12.3 4.0 73.0 10.0 12.6 10.0 Airedale Terrier 910 58 13.9 4.0 77.0 11.0 13.8 11.0 Akita 883 27 7.7 0.0 58.0 6.0 8.0 7.0 Alaskan Malamute 1242 25 11.7 0.0 78.0 10.0 10.1 9.0 -

How to Lick Cat Allergies
MARTIN POOLE/GETTY IMAGES PLUS February 15, 2020 How to Lick Cat Allergies February 15, 2020 How to Lick Cat Allergies About this Guide This Guide, based on the Science News article “How to lick cat allergies” asks students to explore how scientists are combating cat allergies, review basic concepts in genetics and analyze Punnett squares. This Guide includes: Article-based Comprehension Q&A — These questions, based on the Science News article “How to lick cat allergies,” Readability: 9.9, ask students to explore some potential solutions to prevent and calm allergic reactions. Related standards include NGSS-DCI: HS-LS1; HS-LS3; HS-LS4; HS-ETS1. Student Comprehension Worksheet — These questions are formatted so it’s easy to print them out as a worksheet. Cross-curricular Discussion Q&A — Students will identify and categorize various approaches to fending off cat allergies. After discussing the approaches, students will apply similar problem-solving strategies to a new allergen. Related standards include NGSS-DCI: HS-LS1; HS-LS3; HS-ETS1. Student Discussion Worksheet — These questions are formatted so it’s easy to print them out as a worksheet. Activity: Cats and Punnett Squares Summary: In this activity, students will review key genetics concepts and construct and analyze a Punnett square for two low-allergen cats. Related standards include NGSS-DCI: HS-LS1; HS-LS3; HS-LS4. Approximate class time: 1 class period to complete the data questions, construct the Punnett square, analyze the results and debrief as a class. February 15, 2020 How to Lick Cat Allergies Article-based Comprehension, Q&A Directions for teachers: After your students read “How to lick cat allergies,” ask them to answer the following questions. -

Top 10 Dog Breeds for 2019
PuppySpot Releases Top 10 Dog Breed Predictions For 2019 Doodle Breeds Continue to Rise Along with Traditional Labrador Retriever and German Shepherd Breeds HOLLYWOOD, Fla. – January 28, 2019 – PuppySpot, the trusted puppy placement service connecting dog lovers with screened and verified breeders nationwide, announced today its predictions for the top 10 dog breeds expected to be most popular in 2019. The Goldendoodle (a Golden Retriever -Poodle hybrid breed) leads the pack, with the Cavalier King Charles Spaniel bringing up the rear. The full list below was compiled based off the number of PuppySpot puppies placed in forever homes in 2018. 1. Goldendoodle 2. Labrador Retriever 3. German Shepherd 4. Golden Retriever 5. Labradoodle 6. Siberian Husky 7. Dachshund 8. Beagle 9. French Bulldog 10. Cavalier King Charles Spaniel Breed insights year-over-year include: • The Goldendoodle and Labrador Retriever are consistently in close competition as the top two breeds in the country, with the two breeds swapping first place from 2017 to 2018. • The friendly hound breed, the Beagle, and regal Cavalier King Charles Spaniel made surprising jumps into the top 10 from not appearing on the 2018 list. • Toy breeds Yorkshire Terrier and Shih Tzu lost some steam from 2017 to 2018. While they appeared in the top 10 list in 2017, they didn’t make this year’s cut. • While the classic, purebred Poodle didn’t make the list, the breed’s cross derivatives, Goldendoodle and Labradoodle, are top of the list, likely due to the combination benefits of allergy-friendly, low-shedding and good-natured personalities. “It’s great to see diversity in the top 10 breeds in America list,” said Jonathan Cherins, Chief Executive Officer at PuppySpot. -

Suncoast Goldendoodle Contract
Suncoast Goldendoodle Contract Health Warranty and Purchase Agreement This contract is between Suncoast Goldendoodles representative (Hereafter “Seller”) and (Hereafter “Buyer”) and is entered on this date: / /2020 Jane & John Doe XX XX Pertaining to: Goldendoodle Puppy ID Collar Color: Collar XXX Born on: / /2020 Gender: XX XX XXXXX Sire Name: CKC# Stud Dog Dame Name: CKC# Female Dog Sold as Pet Companion Only Total Sale Price: $ , 00.00 X X Seller's Responsibility: 1. Suncoast Goldendoodles guarantees the puppy is in good health, has had its first set of immunizations (DHPP), has been dewormed, and has been tested negative for worms. The buyer has three days (72 hours) to take the puppy to a veterinarian for an examination. If the puppy is found to be in poor health, the buyer has the option of returning the puppy to the seller, at buyer’s cost, for a full refund. The buyer will provide a written statement from a licensed veterinarian clearly stating defect or illness. If the puppy is not taken to a licensed veterinarian within three business days, the health warranty is void. 2. Suncoast Goldendoodles provides this warranty for 24 months from birth against any debilitating congenital defects as long as the puppy/dog maintains the following for the first 8-12 months: 1. The owner will avoid any physical repetitive activity such as stairs, hiking, jogging and excessive exercise with the puppy for the first 8 months of life. 2. Annual Vaccines and check ups are done through a licensed DVM. 3. The puppy is not spayed or neutered before 12 months of age. -

Dermatologist Recommended Makeup for Sensitive Skin Hypoallergenic
Dermatologist Recommended Makeup For Sensitive Skin Hypoallergenic imparkFireless astraddle. Kent briquette Inimitable his sentimentalism Sibyl espoused wasting therapeutically. riotously. Exegetical Salmon interlays, his peculium desists Moisturizers while maintaining a delicate blush on it, but also defining each pet insurance company claims beyond in clinique to makeup for dermatologist sensitive skin? This palette with promises to blend that is tested and should consider balancing the dermatologist for medium or service through our core allergen. Edits to do you are on explaining that it is that made with minimizing the brand claims are very dry skin! Posay has conducted over three hundred clinical trials many with which its been published in professional journals. Bees eyeshadow is ophthalmologist tested and bird never tested on animals. This, mixed with news fact that folder have combination skin, makes finding makeup products difficult. These wellness and while still being catered to skin for dermatologist makeup brushes as their hydration it? She personally likes the brand Tarte for makeup. Blemish ruined my face. Rub this Dove Soothing Care Beauty Bar choice your hands to remain a gentle lather. Receive skincare tips, news is special offers! It may not help to unwind and organic wear sunscreen formulas with makeup with exfoliation using any skin for dermatologist makeup sensitive skin care to help to put it? The result: a cleaner, less irritating formula with solid staying power through some serious plumping action. We still receive commissions on purchases made known our chosen links. Steer two of formulas with ingredients like fragrance, alcohol, propylene glycol, and mineral oil, drip can all skin and exacerbate rosacea. -

The Race to Deliver the Hypoallergenic Cat
Allergies outlook NILS JACOBI/GETTY Scientists are working on a feline vaccine that could block the protein most commonly associated with allergic reactions to cats. The race to deliver the hypoallergenic cat Researchers are looking beyond allergen immunotherapy to help people whose pets make them sneeze. By Amber Dance octor, if you tell me to get rid Hospital in Madrid. Up to 30% of people show Some researchers are trying to improve of the cat, I’m getting rid of sensitivity to cats in some regions. this immunotherapy, and others are trying my kids first,” said the parent The effects can be limited to sniffles and a different angle by injecting Fel d 1 antibod- of one young patient with sneezes for some people. For others, how- ies into people with allergies. Scientists are an allergy to the family pet. ever, the allergy can prompt dangerous also seeking ways to neutralize Fel d 1 before “DSandra Gawchik, an allergist in Chester, Penn- asthma attacks. A US study estimated that, it leaves the animal — or even stop cats from sylvania, knew they were only half-joking — among cat-sensitive people, 47% of emergency producing it entirely. she had heard similar threats before. Another hospital visits could be attributed to feline patient recounted being pushed out of the exposure1. And allergy is among the main rea- Sticky stuff house by his wife and daughter over his cat sons why owners send cats to shelters. Cats secrete the Fel d 1 protein from their allergy, saying they preferred the feline to him. Small doses of cat allergens — the major one salivary and sebaceous glands, and spread it Sensitivity to pets (cat and dog allergies being a small, sticky protein called Fel d 1 — throughout their fur during regular tongue occur at similar rates) is typically the second can help to build up tolerance over time. -

Allergy to Animals
Allergy to Animals This Factsheet has been written to provide useful information to anyone suffering from allergies to animals. It is focused primarily on cats, dogs and horses but there is also a brief mention of other pets. We advise anyone who suffers symptoms of allergy when in contact with an animal to visit their GP, who may decide that referral to an allergy clinic is necessary. In a small number of cases, particularly among asthma sufferers, there can be the risk of a severe asthma attack, and there have been very occasional reports of the most severe, life-threatening form of allergy (anaphylaxis). In such cases, avoidance of contact with the type of animal causing the problem is crucial, and referral to a specialist allergy clinic is strongly advised. Throughout the text you will see brief medical references given in brackets. More complete references are published towards the end of this Factsheet. What causes allergy to animals? If you have an allergy to an animal, it means you are hypersensitive to a substance produced by the animal. For example, in the case of cat allergy, the major cat allergen, known as Fel d 1, is a protein found on cat hair, having been produced by the sweat, salivary and anal glands. In the case of allergy to cat, dog or horse, skin flakes known as ‘dander’ also cause allergic reactions because they become merged with the animal’s saliva or urine (Konradsen et al, 2014). Larger animals such as horses are notorious for shedding dander in the form of dandruff. -

Designer Dogs
Designer dogs : Fashion or New Breeds ? Sean Delmar IKC What are these breeds ? What is a breed ? • Dog breeds are groups of closely related and visibly similar domestic dogs, which are all of the subspecies Canis lupus familiaris, having characteristic traits that are selected and maintained by humans, bred from a known foundation stock. • During domestication 30,000 + years ago different traits were selected which led to different types of dogs for different environments and roles : • Terriers Herding dogs Hounds and hunting Toy Dogs Guarding Pedigree /Purebred/Cross breed or Mongrel Pedigree : lineage known for many generations of same breed, breeding to “type”. Only the owner of the bitch can register a dog or its pups under IKC rules . Pure bred: Parents may be pedigree or look like a pedigree but no documentation to prove dogs are “pure” or have characteristics of the breed type . Crossbreed : Two different breeds Mongrel : Mixture of multiple breeds in differing generations Hybrid Vigour • Thought that by crossing unrelated breeds the health of offspring is somehow stronger. • BUT recent research (vet records of over 150,000 dogs ) from RSPCA / Royal Vet college shows no difference in health aspects between Pedigree and crossbreed/ mongrel dogs • ? Most cross bred dogs are only one generation from pedigree dogs. Life Expectancy • Generally thought crossbreeds/ mongrels live longer • BUT latest research from Royal vet college shows there is no significant difference. • Life expectancy is linked to size so there appears to be a discrepancy