Handbook of Thermoplastics Polyvinyl Butyral
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2 Types of Noncrystalline Polymers
2 Types of Noncrystalline Polymers 1. Glassy polymer highly interpenetrated/entangled 2. Rubbery polymers random Gaussian coils Glass Transition Temperature εij Two viewpoints: • Increasing T: When kT > magnitude of εij, the thermal fluctuations can overcome local intermolecular bonds and the frozen (“glassy”) structure becomes “fluid-like”. • Decreasing T: As the temperature is lowered and T approaches Tg, the viscosity increases to ∞ and the material becomes “solid” Free Volume Theory of Tg Free volume, VF – extra space beyond what is present in an ordered crystalline packing (beyond the interstitial volume). VF(T) ≡ V(T) – V0(T) • V0 is occupied specific volume of atoms or molecules in the xline state and the spaces between them: ~ VXL. V(T) α • VF increases as T increases due to the Rate of cooling l difference in the thermal expansion V (T) αg XL coefficients (αg vs αl). FAST SLOW • V0(T) ≈ VXL(T) ↔ can take αg ≈αXL dV •V(T) = V (T ) + (T-T ) F T > T F F g g dT g Tg T • define fractional free volume, fF:Vf/V fF(T) = fF(Tg) + (T-Tg)αf αf = αl – αg Viewpoint: Tg occurs when available free volume drops below critical threshold for structural rearrangement [VITRIFICATION POINT], structure “jams up”. Tg Values of Amorphous Materials Table of representative amorphous solids, their bonding types, and their glass transition temperatures removed due to copyright restrictions. See Table 2.2 in Allen, S. M., and E.L. Thomas. The Structure of Materials. New York, NY: J. Wiley & Sons, 1999. Tg for Selected Polymers Glass Transition Temperature -

"3-47-2. 2,720,501 United States Patent Office Patented Oct
Oct. 11, 1955 R. T. VAN NESS 2,720,501 AQUEOUS CONDENSATION PROCESS FOR THE PREPARATION OF POLY WINYL ACETAL RESINS Filed Aug. 10, 1954 ALDEHYDE-f f 27 INVENTOR Robert Terry Van Ness "3-47-2. 2,720,501 United States Patent Office Patented Oct. 11, 1955 2 alcohol groups, and the remainder is polyvinyl butyral. In the attached drawing there is illustrated in a sche 2,720,501 matic fashion a flow sheet representing the process of AQUEOUS CONDENSATIGN PROCESS FOR THE this invention. A solution of polyvinyl alcohol in water PREPARATION OF POLYWNYLACETAL RESNS is prepared in vessel which is fitted with a means for agitating and a means for heating the contents of the Robert Terry Van Ness, Wilmington, Del, assignor to vessel. Polyvinyl alcohol from a storage vessel 3 is E. I. du Pont de Nemours and Company, Wimington, added to vessel 1 along with sufficient cold water at a Del, a corporation of Delaware temperature from about 20° C. to 30° C. to form a sus Application August 10, 1954, Serial No. 448,876 10 pension containing about 7 to about 15% solid polyvinyl alcohol. Polyvinyl alcohol is prepared by hydrolyzing 4 Claims. (CI. 260-73) polyvinyl acetate in the presence of an alkaline catalyst or an acid catalyst. Depending upon the polyvinyl ace tate used, the type of hydrolysis catalyst, and the de This invention relates to the preparation of polyvinyl 5 gree to which the hydrolysis is completed, many varieties acetal resins and, more particularly, it relates to a proc of polyvinyl alcohol may be prepared. -

US5147908.Pdf
|||||||||||||||| USOO5147908A United States Patent (19) 11 Patent Number: 5,147,908 Floyd et al. 45) Date of Patent: Sep. 15, 1992 (54) CATIONICPOLYVINYL ALCOHOL BINDER 4,405,375 9/1983 Gibson et al........................ 106/277 ADDITIVE 4,461,858 7/1984 Adelmann ........ ... 524/503 4,528,316 7/1985 Soerens ........ ... 524/503 75) Inventors: William C. Floyd, Chester; Louis R. 4,537,807 8/1985 Chan et al. ............................ 428/74 Dragner, Rock Hill, both of S.C. 4,816,540 3/1989 Onishi .......... ... 527/300 4,837,087 6/1989 Floyd et al. ......................... 428/51 73 Assignee: Sequa Chemicals Inc., Chester, S.C. 4,888,386 12/1989 Huang et al. ... ... 524/503 X (21) Appl. No.: 587,012 4,954,577 9/1990 Dinwald et al. ............... 524/503 X 22 Filed: Sep. 24, 1990 OTHER PUBLICATIONS 51) Int. Cl. ................................................ CO8L 3/04 Zunker, David W.; "Incorporating The Benefits Of 52 U.S. Cl. ........................................ 524/49; 524/55; Polyvinyl Alcohol At the Wet-End Of Papermaking'; 524/503; 525/57; 525/58 1982 Papermakers Conference. 58 Field of Search ..................... 524/503, 49, 55, 57, 524/58 Primary Examiner-Joseph L. Schofer Assistant Examiner-J. M. Reddick 56) References Cited Attorney, Agent, or Firm-Mitchell D. Bittman U.S. PATENT DOCUMENTS 57 ABSTRACT 3,573,236 3/1971 Barlow .....................r 260/7 3,597,313 8/1971 Williams et al. ... ... 162/67 A cationic polyvinyl alcohol binder additive is prepared 3,600,342 8/97 Nickerson et al. ................... 260/17 suitable for addition in the wet-end of a paper making 3,700,61 0/1972 Nickerson et al. -
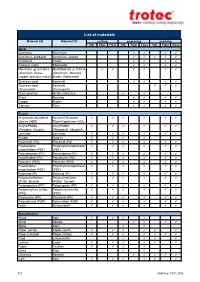
List of Materials
List of materials Material EN Material DE cutting engraving marking CO2 Fiber Flexx CO2 Fiber Flexx CO2 Fiber Flexx Metal Aluminum Aluminium Aluminum, anodized Aluminium, eloxiert Chromium Chrom Precious metal Edelmetall Metal foils up to 0.5mm Metallfolie bis zu 0,5mm (Aluminum, Brass, (Aluminium, Messing, Copper, precious metal) Kupfer, Edelmetall) Stainless steel Edelstahl Stainless steel Edelstahl (Thermark®) (Thermark®) Metal, painted Metall, lackiertes Brass Messing Copper Kupfer Titanium Titan Plastic Acrylonitrile butadiene Acrylnitril-Butadien- styrene (ABS) Styrol-Copolymer (ABS) Acrylic/PMMA Acryl/PMMA (Plexiglas, Altuglas, (Plexiglas®, Altuglas®, LaminatePerspex) LaminatePerspex®) Rubber Gummi Polyamide (PA) Polyamid (PA) Polybutylene Polybutylenterephthalat terephthalate (PBT) (PBT) Polycarbonate (PC) Polycarbonat (PC) Polyethylene (PE) Polyethylen (PE) Polyester (PES) Polyester (PES) Polyethylene Polyethylenterephthalat terephthalate (PET) (PET) Polyimide (PI) Polyimid (PI) Polyoxymethylene Polyoxymethylen (POM) -Delrin® (POM) - Delrin® Polypropylene (PP) Polypropylen (PP) Polyphenylene sulfide Polyphenylensulfid (PPS) (PPS) Polystyrene (PS) Polystyrol (PS) Polyurethane (PUR) Polyurethan (PUR) Foam Schaumstoff Miscellanious Wood Holz Mirror Spiegel Stone Stein Paper (white) Papier (weiß) Paper (colored) Papier (färbig) Food Lebensmittel Leather Leder -

Designing with Laminated Glass
Laminated Glass Railings with DuPont Interlayers Valerie Block, Senior Marketing Specialist DuPont Glass Laminating Solutions DuPont Glass Laminating Solutions The DuPont Company has supplied plastic ‘interlayers’ for manufacture of laminated safety glass since the advent of safety glass for the car windshield beginning with Ford in 1924. Interlayer chemistry progressed from cellulose nitrate to cellulose acetate and then to PVB plasticized interlayer which was honored in 1938 as a major technological achievement. The latest major interlayer development for laminated glass by DuPont in 1998 was DuPont launches Butacite® PVB ® SentryGlas structural interlayer. interlayer for safety glass - 1938 Laminated glass • Two or more lites of glass and one or more interlayers – Polyvinyl butyral (PVB) – Ionoplast A sandwich of “glass” and “plastic” Who makes it? Glass Interlayer supplier supplier Glass fabricator Glazing System contractor manufacturer The Laminating Process Nip rolls Glass washer Lay up Ovens Unloading area Laminated glass offers … • Safety • Glass retention • Durability • Cleaning ease • Missile impact performance • Decorative options Types of Glass • Annealed (basic glass) • Patterned • Tempered* (4x the strength of annealed) • Heat strengthened* (2x the strength of annealed) • Curved *Polishing of glass edges is done prior to heat- treating Safety • Safety after breakage • Interlayers retain glass fragments if breakage occurs • Laminated glass is retained in frame • Meets safety glazing impact requirements Glass retention • Safety after breakage • Interlayers retain glass fragments if breakage occurs • Laminated glass is retained in frame Durability • Laminates go through natural and accelerated testing for weathering to demonstrate durability Cleaning Ease • Glass surfaces are easy to clean with readily available cleaning products • Glass surfaces are durable-no yellowing, cracking, or crazing Missile Impact Performance • Large Missile Test – Covers the first three floors – 9 lb. -

A Finite Element Model for Impact Simulation with Laminated Glass
ARTICLE IN PRESS International Journal of Impact Engineering 34 (2007) 1465–1478 www.elsevier.com/locate/ijimpeng A finite element model for impact simulation with laminated glass M. Timmela, S. Kollingb,Ã, P. Osterriederc, P.A. Du Boisd aUniversity of Leipzig, Institute for Structural Mechanics, Marschnerstr. 31, 04109 Leipzig, Germany bDaimlerChrysler AG, EP/SPB, HPC X271, 71059 Sindelfingen, Germany cUniversity of Cottbus, Universita¨tsplatz 3-4, 03044 Cottbus, Germany dFreiligrathstr. 6, 63071 Offenbach, Germany Received 15 January 2004; received in revised form 10 March 2006; accepted 13 July 2006 Available online 8 January 2007 Abstract A computational technique for the modelling of laminated safety glass is presented using an explicit finite element solver. Coincident finite elements are used to model the layered set-up of laminated glass: shell elements with brittle failure for the glass components and membrane elements to simulate the ultimate load carrying capacity of the PVB-interlayer. Two different approaches are considered to model laminated glass: a physical model and a smeared model. In the physical model the glass is considered as elastic/brittle and the interlayer as a hyperelastic material. For the hyperelastic description of the interlayer, we give an overview of material models, which are widely used for explicit solvers, i.e. the laws by Blatz–Ko, Mooney–Rivlin and Ogden. The obtained stress–strain curves are fitted to experimental results of the interlayer. The hyperelastic model is applied to a simple impact test demonstrating the numerical robustness. In the smeared model, we use two shell elements of equal thickness with elasto-plastic material properties to obtain an improved bending response after fracture. -

Use of a Crosslinking Agent, Crosslinked Polymer, and Uses Thereof
(19) TZZ Z_T (11) EP 2 452 970 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C08K 5/09 (2006.01) B32B 27/30 (2006.01) 21.01.2015 Bulletin 2015/04 C08L 29/04 (2006.01) C08L 101/00 (2006.01) G02B 5/30 (2006.01) C08K 5/098 (2006.01) (2006.01) (21) Application number: 12154871.3 C08K 5/10 (22) Date of filing: 29.08.2008 (54) Use of a crosslinking agent, crosslinked polymer, and uses thereof Verwendung eines Vernetzungsmittels, vernetztes Polymer und Verwendungen davon Utilisation d’un agent de réticulation, polymère réticulé et utilisations associées (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Kageyama, Hideki HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT Osaka-shi, Osaka 530-0018 (JP) RO SE SI SK TR • Tanaka, Shinichi Osaka-shi, Osaka 530-0018 (JP) (30) Priority: 31.08.2007 JP 2007225012 • Ono, Hiroyuki 18.03.2008 JP 2008069645 Osaka-shi, Osaka 530-0018 (JP) 04.04.2008 JP 2008098282 • Kuruma, Akiko 11.04.2008 JP 2008103494 Osaka-shi, Osaka 530-0018 (JP) 24.04.2008 JP 2008114149 24.04.2008 JP 2008114150 (74) Representative: Kuhnen & Wacker Patent- und Rechtsanwaltsbüro (43) Date of publication of application: Prinz-Ludwig-Straße 40A 16.05.2012 Bulletin 2012/20 85354 Freising (DE) (62) Document number(s) of the earlier application(s) in (56) References cited: accordance with Art. 76 EPC: WO-A1-2005/087711 JP-B2- 3 612 124 08828502.8 / 2 189 496 US-A1- 2004 102 586 (73) Proprietor: The Nippon Synthetic Chemical • DATABASE WPI Week 198020 Thomson Industry Co., Ltd. -

BASEL CONVENTION PLASTICS AMENDMENTS Institute of Scrap Recycling Industries (ISRI)
BASEL CONVENTION PLASTICS AMENDMENTS Institute of Scrap Recycling Industries (ISRI) Adina Renee Adler Assistant Vice President, International Affairs ©2019 Institute of Scrap Recycling Industries, Inc. All Rights Reserved The Driver 2 Basel Convention Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal Adopted 22 March 1989 Entered into force on 5 May 1992 Currently has 187 parties The United States is not a party. OCTOBER 2019 | ISRI.ORG 3 ©2019 ISRI, Inc. Basel Convention Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal Subjects hazardous wastes or other wastes with hazardous constituents / exhibiting hazardous characteristics to a “prior informed consent” (PIC) procedure before the material can be shipped across national borders. The PIC gives the receiving country the right of refusal if proper recycling or disposal operations do not exist. OCTOBER 2019 | ISRI.ORG 4 ©2019 ISRI, Inc. Basel Convention 14th Conference of the Parties (COP) held every two years to direct work on implementing the convention and addressing emerging issues. OCTOBER 2019 | ISRI.ORG 5 ©2019 ISRI, Inc. Norway’s Proposal Amend the following annexes so that trade controls are 1 put into place for certain plastics: Annex II: Non-hazardous, hard-to-recycle plastics Annex VIII: Hazardous plastics Annex IX: Non-hazardous, easy-to-recycle plastics (exempt from BC) Create a Partnership for Plastic Waste that would involve public and private stakeholders to find possible solutions 2 to plastic leakage. Terms of Reference negotiated and adopted. It will lead to non- binding initiatives to support developing country improvements to minimization, collection and proper handling of end-of-life plastics. -

Removal of Anionic Dye Congo Red from Aqueous Environment Using
www.nature.com/scientificreports OPEN Removal of anionic dye Congo red from aqueous environment using polyvinyl alcohol/sodium alginate/ ZSM‑5 zeolite membrane Sabarish Radoor1*, Jasila Karayil2, Jyotishkumar Parameswaranpillai1 & Suchart Siengchin1* In this study, a novel PVA/SA/ZSM‑5 zeolite membrane with good regeneration capacity was successfully prepared by solvent casting technique. The properties of the membranes were assessed by employing diferent characterization techniques such as X‑ray difraction (XRD), Fourier‑transform infrared spectroscopy (FT‑IR), scanning electron microscope (SEM), optical microscopy (OP), thermogravimetric analysis (TGA), contact angle and universal testing machine (UTM). XRD, TGA and UTM results revealed that the crystallinity and thermo‑mechanical performance of the membrane could be tuned with zeolite content. The successful incorporation of zeolite into the polymer matrix was confrmed by FT‑IR, SEM and OP analysis. The adsorption ability of the as‑prepared membrane was evaluated with a model anionic dye, Congo red. Adsorption studies show that the removal efciency of the membrane could be tuned by varying zeolite content, initial concentration of dye, contact time, pH and temperature. Maximum dye adsorption (5.33 mg/g) was observed for 2.5 wt% zeolite loaded membrane, at an initial dye concentration of 10 ppm, pH 3 and temperature 30 °C. The antibacterial efciency of the membrane against gram‑positive (Staphylococcus aureus) and gram‑negative bacteria (Escherichia coli) was also reported. The results show that membrane inhibits the growth of both gram‑positive and gram‑negative bacteria. The adsorption isotherm was studied using two models: Langmuir and Freundlich isotherm. The results show that the experimental data ftted well with Freundlich isotherm with a high correlation coefcient (R2 = 0.998). -

Crosslinked Poly(Vinyl Alcohol) Membranes
International Journal of ChemTech Research CODEN (USA): IJCRGG, ISSN: 0974-4290, ISSN(Online):2455-9555 Vol.10 No.3, pp 118-127, 2017 Synthesis and Physical-Chemical Characterization of DEA- Crosslinked Poly(Vinyl Alcohol) Membranes María T. Acevedo1*, Alejandra Tapia1, Álvaro Realpe1 1Department of Chemical Engineering, Research Group of Modeling of Particles and Processes, Engineering Faculty, Universidad de Cartagena, Colombia Abstract : In this research, crosslinked PVA was impregnated with 0, 15 and 20% of diethanolamine so as to study the effect of the alkanolamine on the physical-chemical properties which are connected to the CO2 separating process via membranes. Formaldehyde and a porous membrane of polisulfone were used as crosslinking agent and support, respectively. Membranes were characterized by FTIR, SEM-EDS, contact angle, water absorption and porosity measurements. The results suggest that although the water absorption in the bulk of polymer diminished when the percentage of diethnaolamine increased, the surface hydrophilicity and appearance of the membranes were not appreciably affected due to this modification. A favorable balance of properties such as water uptake, the porosity and the superficial hidrophilicity were achieved using the crosslinking and synthesis conditions proposed in this paper, considering the positive effect of those characteristics in the permeability/selectivity of CO2 through the membrane. Keyword : crosslinked PVA, diethanolamine, CO2 separating process, membranes, surface hydrophilicity. Introduction Pollution associated with emissions of carbon dioxide (CO2) is one of the preeminent environmental issues [1,2]. The high level of CO2 emission levels is arising from combustion of fossil fuel, accelerated increase in the energy consumption, population and industries, including cement and metal production, which have caused different kinds of devastating effects in particular the increase in global temperature with severe consequences on ecosystems, biodiversity and sea levels [3]. -

Crosslinking of Poly(Vinyl Alcohol) Via Bis(\Beta-Hydroxyethyl) Sulfone
Polymer Journal, Vol. 36, No. 6, pp. 472—477 (2004) Crosslinking of Poly(vinyl alcohol) via Bis( -hydroxyethyl) Sulfone y Kazuhiro KUMETA,Ã;ÃÃ Ichiro NAGASHIMA,ÃÃ Shigetoshi MATSUI,Ã and Kensaku MIZOGUCHIÃ; ÃDepartment of Materials Science and Chemical Engineering, Shizuoka University, 3-5-1 Johoku, Hamamatsu 432-8561, Japan ÃÃNitivy Co., 300 Zenzaemon, Fujieda 426-0053, Japan (Received November 4, 2003; Accepted March 29, 2004; Published June 15, 2004) ABSTRACT: Poly(vinyl alcohol) (PVA) was crosslinked via bis( -hydroxyethyl) sulfone (BHES) at elevated tem- peratures to improve the water resistance. The best conditions for the crosslinking such as temperature, heat-treatment period, and amount of BHES or catalyst were screened out. Water resistance of the crosslinked PVA was characterized in terms of swelling and weight loss in water. The addition of BHES into PVA followed by heating at elevated temper- ature resulted in successful formation of crosslinking structure. The crosslinking occurs above 160 C and takes shorter period at high temperature than low temperature. The crosslinking catalyst, sodium carbonate, acts more effectively at 160 C than at 200 C. The results from thermal analysis using a differential scanning calorimetry revealed that the cat- alyst functions against BHES to lower the temperature for the crosslinking reaction and that even the crosslinked films have the PVA crystalline. The crosslinking procedure using BHES and sodium carbonate is applicable to form ion-ex- change fibers based on PVA with high water resistance. [DOI 10.1295/polymj.36.472] KEY WORDS Poly(vinyl alcohol) / Crosslinking / Thermal Post-treatment / Bis( -hydroxyethyl) Sulfone / PVA is a typical hydrophilic polymer and can be plied heat-treatment methods to crosslinking PVA readily blended with additives such as particles or oth- via glyoxal.3 In this case, the process requires an acid- er polymers. -

Polyvinylbutyral HVB-0001
Technical Information Polyvinylbutyral HVB-0001 Description: HVB-0001 is high viscosity thermoplastic Polyvinylbutyral Resin combining a very large surface area with medium molecular weight. Its sensorial properties are outstanding. The product is soluble in solvents like Alcohols or Glycol Ethers, it is compatible with Esters and Ketones. HVB-0001 shows excellent compatibility with other resins like Nitrocellulose, Epoxy Resins, Epoxy Alkyds, Phenolic Resins, Polyurethanes, Melamine-Formaldehyde Resins, Ketonic Resins and Polyamides. With its free hydroxyl groups the material is chemically reactive. Application: Printing Inks and lacquers: HVB-0001 is an ideal choice for formation of printing inks for flexible packaging (Flexographic & Gravure application). The product offers good water resistance combined with good blocking resistance, excellent pigment wetting and good flow. It shows good adhesion to substrates like Cellulose Acetate, Polyester, Polystyrene or Metalized Films. HVB-0001 is suitable for food packaging applications. Wash Primers: HVB-0001 can be used for formulation of wash primers for steel storage tanks, bottom of ships, airplane, bridges, dam locks, trailers, farm equipment, automotive, rail coaches etc. The material offers flexible films with good adhesion to metal surfaces. Adhesives: HVB-0001 acts as a plasticizer for adhesive formulations based on Epoxy, Phenolic, Melamine-Formaldehyde Resins and Isocyanates. It helps improving interfacial adhesion, flexibility, impact strength and resistance against solvent, chemicals or high temperature. Ceramics: HVB-0001 solution can be used as a temporary binder for ceramic powder. Typically 2 – 4 % PVB-resin are added to Ceramic. During sintering process HVB-0001 undergoes thermal degradation leaving virtually no residue. It leads to very good green strength and dimensional stability.