Mistletoe and the Brushtailed Possum in Silver Beech Forest, South
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Full Article
Quarterly Bulletin of The Ornithological Society of New Zealand Volume 7, Number Seven :January l 958 NOTORNIS In continuation of New Zealand Bird Notes BULLETIN OP THE ORNITHOLOGICAL SOCIETY OF NEW ZBALAND (Incorporated) Registered with the G.P.O., Wellington, as a Magazine Edited by R. B. SIBSON, King's College, Auckland S.E.7 Annual Subscription, 10/- (Juniors, 5/-); Endowment Membership, Cl; Life Membership, E10 (for members over thirty years of age). OFFICERS, 1957 - 58 President - MR P. C. BULL, Lower Hutt. North Island Vice-President - MR E. G. TURBOTT, Christchurcb South Island Vice-President - MRS L. E. WALKER, Dunedin Editor- MR R. B. SIBSON, King's College, Auckland S.E.7 Treasurer - MR H. R. McKENZIE, North Road, Clevedon Secretary - MR G. R. WILLIAMS, Wildlife Division, Department of Internal Affairs, Wellington MRS 0. SANSOM, Invercargill; DR R. A. FALLA, Wellington; MR J. C. DAVENPORT, Auckland Contents of Volume 7, Number 7 : January 1958 Some Notes on Muttonbirding in the North Island- W. J. Phillipps 189 Classified Summarised Notes .................................... 191 Annual Locality Reports for Firth of Thames and Manukau Harbour 201 Obituary: W. R. B. Oliver ....................................205 Short Notes mentioning: S.I. Pied Oystercatcher, White-faced Heron, Spotted Shag, Barn Owl, Spur-winged Plover, Crested Grebe, 'Red- legged ' Herons, Myna in !;.I., Bush-hawk, Weka ................ 206 Review8 .................................................... 2 11 Notices. XIIth International Ornithological Congress ............ 212 Nest Records Scherne Publications for sale Donations NOTORNI S VOLUME SEVEN NUMBER SEVEN : JANUARY NINETEEN FIFTY-EIGHT SOME NOTES ON MUTTONBIRDIING IN THE NORTH ISLAND By W. 1. PHILLIPPS During the period 1919- 1924 odd notes were collected on the occurrence of muttonbirds breeding on Mount Pihanga not far from Lake Rotoaira. -
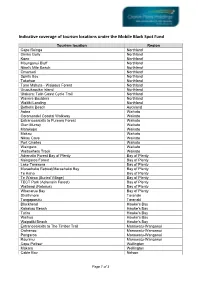
Indicative Coverage of Tourism Locations Under the Mobile Black Spot Fund
Indicative coverage of tourism locations under the Mobile Black Spot Fund Tourism location Region Cape Reinga Northland Glinks Gully Northland Kaeo Northland Maunganui Bluff Northland Ninety Mile Beach Northland Omamari Northland Spirits Bay Northland Takahue Northland Tane Mahuta - Waipoua Forest Northland Urupukapuka Island Northland Utakura: Twin Coast Cycle Trail Northland Wairere Boulders Northland Waitiki Landing Northland Bethells Beach Auckland Aotea Waikato Coromandel Coastal Walkway Waikato Entrances/exits to Pureora Forest Waikato Glen Murray Waikato Marokopa Waikato Mokau Waikato Nikau Cave Waikato Port Charles Waikato Waingaro Waikato Waitawheta Track Waikato Adrenalin Forest Bay of Plenty Bay of Plenty Kaingaroa Forest Bay of Plenty Lake Tarawera Bay of Plenty Maraehako Retreat/Maraehako Bay Bay of Plenty Te Kaha Bay of Plenty Te Wairoa (Buried Village) Bay of Plenty TECT Park (Adrenalin Forest) Bay of Plenty Waitangi (Rotorua) Bay of Plenty Whanarua Bay Bay of Plenty Strathmore Taranaki Tongaporutu Taranaki Blackhead Hawke's Bay Kairakau Beach Hawke's Bay Tutira Hawke's Bay Waihua Hawke's Bay Waipatiki Beach Hawke's Bay Entrances/exits to The Timber Trail Manawatu-Wanganui Owhango Manawatu-Wanganui Pongaroa Manawatu-Wanganui Raurimu Manawatu-Wanganui Cape Palliser Wellington Makara Wellington Cable Bay Nelson Page 1 of 3 Kenepuru Head Marlborough Okiwi Bay Marlborough Blue Lake/ Lake Rotoroa Tasman Cape Farewell Tasman Entrances/exits to Heaphy Track Tasman Lake Rotoroa Tasman Maruia Falls Tasman Totaranui Beach and campsite -

Haast Regional Walks Brochure
Mäori first settled here at least 800 years ago, the sea, Haast Visitor Centre Introduction coast and navigable rivers providing main points of access. Mäori settlement and activity was centred around Information on the Te Wähipounamu - South West New The Haast area is more than a collection of small gathering, carving and trading precious jade, known as Zealand World Heritage Area, other lands administered by settlements near the main highway or along the road to pounamu (greenstone). Jackson Bay Okahu. It is a diverse region, stretching the Department of Conservation, tracks, accommodation European settlement was attempted at Jackson Bay Okahu from Knights Point to the Cascade Valley and inland to the and advice on recreational opportunities in the Haast area during the 1870s. The pioneers’ attempt to “tame” the forest-lined Haast Pass. The area offers a wide variety of can be obtained from the Haast Visitor Centre at Haast landscape was largely unsuccessful but their efforts left scenery, chances to view wildlife and many recreational (situated on the corner of SH6 and the Jackson Bay Road). a tradition of South Westland residents as being tough, opportunities. Hut tickets, hunting permits, maps, conservation souvenirs resilient and independent. and publications can also be obtained from the visitor The region is famous for it’s dramatic coastline - the This brochure should help visitors find their way around the centre. EFTPOS is available. sweeping curves of beaches, the rugged cliff tops, and Haast area. Displays at the Department of Conservation’s the striking rock formations at Knights Point south of Lake Haast Visitor Centre and at other sites within the World Moeraki. -

Peraxilla Colensoi
Peraxilla colensoi COMMON NAME Scarlet mistletoe, korukoru, pirita, roeroe SYNONYMS Elytranthe colensoi (Hook.f.) Engl. Loranthus colensoi Hook. f. FAMILY Loranthaceae AUTHORITY Peraxilla colensoi (Hook.f.) Tiegh. FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes ENDEMIC GENUS Yes Peraxilla colensoi, Catlins. Photographer: John Barkla ENDEMIC FAMILY No STRUCTURAL CLASS Trees & Shrubs - Dicotyledons NVS CODE PERCOL CHROMOSOME NUMBER 2n= 24 CURRENT CONSERVATION STATUS 2012 | At Risk – Declining | Qualifiers: CD PREVIOUS CONSERVATION STATUSES 2009 | At Risk – Declining | Qualifiers: CD 2004 | Gradual Decline BRIEF DESCRIPTION Fleshy shrub to 3m wide growing on outer branches of beech trees with glossy green fleshy paired leaves and masses of red tubular flowers. Leaves to 8cm long, smooth with a red edge. Flowers to 2.5cm long. Fallen petals litter forest floor under plants. Fruit yellow. Photographer: Brian Molloy DISTRIBUTION North and South Island, but common only in southern parts of the South Island. HABITAT A parasite mainly found in silver beech forest but has been recorded on 16 host species (9 exotic) in New Zealand including red beech and black beech. Tui (Prosthemadera novaeseelandiae) and bellbird (Anthonis melanura) disperse this species in the North Island. FEATURES A shrub up to 3 m across. It parasitises further out on branches of its host than Peraxilla tetrapetala. The veins on leaves are hardly evident and only the midrib is conspicuous. Leaf tips are never notched and the leaves themselves are large and never blistered. The leaves sit in pairs on opposite sides of the stem and are thick and have a leathery texture. Leaf margins are usually smooth with red slightly rough margins. -

Explosive New Zealand Mistletoe Example, 4 of 7 Flowers on Sheet AK103910)
SCIENTIFIC CORRESPONDENCE pattern, as do herbarium sheets (for Explosive New Zealand mistletoe example, 4 of 7 flowers on sheet AK103910). Trilepidea almost certainly SIR- Many flowers of the mistletoe arium sheets show an even more special had more complex explosive flowers than Peraxilla tetrapetala (Loranthaceae) in New ized explosive mechanism than in Peraxilla. Peraxilla. Such specialization may have Zealand open from the bottom (f in the We discovered explosive flower opening rendered Trilepidea more sensitive to figure) rather than the top (d); Kuijtl called in both endemic New Zealand Peraxilla reduced bird densities due to introduced this an "unsolved mystery ... we cannot species when we noted that flower buds mammalian predators, contributing to its even guess at the meaning of this bizarre bagged for hand pollination almost never rapid and puzzling5 decline. performance." Here we report that flower opened (3/394 for P. tetrapetala, 1/82 for P. Explosive flower opening is well known buds of P. tetrapetala and P. colensoi open colensoi). The petals remained fused at the in other mistletoes6, including many of from the top only when twisted by top, while eventually undergoing abscis the 230 species in Africa3, and a few a bird, a form of 'explosive' flower open sion from their base (fin the figure). Field species in India7, Java, New Guinea and ing common in Africa but previously observations of unbagged flowers revealed South America1•6. However, this is the unknown in Australasia. Kuijt's "bizarre that they were opened by two native first report from Australasia. The New performance" is simply the consequence of honeyeaters (Meliphagidae ): tuis (Pros Zealand flora generally displays few flowers not being visited by birds. -

Franz Josef Glacier Township
Mt. Tasman Mt. Cook FRANZ JOSEF IMPORTANT PHONE NUMBERS www.glaciercountry.co.nz EMERGENCY Dial 111 POLICE (Franz Josef) 752 0044 D Franz Josef Health Clinic 752 0700 GLACIER TOWNSHIP Glacier The Visitor Centre at Franz Josef is open 7 days. I After hours information is available at the front I I entrance of the Visitor Centre/DOC offi ce. H Times given are from the start of track and are approximate I 1 A A. GLACIER VALLEY WALK 1 1 hour 20 mins return following the Waiho riverbed 2 20 G B to the glacier terminal. Please heed all signs & barriers. 14 B. SENTINEL ROCK WALK Condon Street 21 C 3 15 24 23 20 mins return. A steady climb for views of the glacier. 5 4 Cron Street 16 C. DOUGLAS WALK/PETERS POOL 25 22 43 42 12 26 20 mins return to Peter’s Pool for a fantastic 13 9 6 31 GLACIER E refl ective view up the glacier valley. 1 hour loop. 11 7 17 30 27 45 44 10 9 8 Street Cowan 29 28 ACCESS ROAD F D. ROBERTS POINT TRACK 18 33 32 Franz Josef 5 hours return. Climb via a rocky track and 35 33 State Highway 6 J Glacier Lake Wallace St Wallace 34 19 Wombat swingbridges to a high viewpoint above glacier. 40 37 36 Bus township to E. LAKE WOMBAT TRACK 41 39 38 Stop glacier carpark 40 State Highway 6 1 hour 30mins return. Easy forest walk to small refl ective pond. 46 is 5 km 2 hour F. -

NEW ZEALAND NOTES·, 1954-5 by DAVID HALL
• NEW ZEALAND NOTES, 1954-5 39 1 . NEW ZEALAND NOTES·, 1954-5 By DAVID HALL HE last climbing season was notable for two things : first, the early disappearance of snow providing in early January conditions which might have been expected six weeks later ; second, the high death roll in accidents. A consequence of the lack of snow was that much rock was exposed which would have been concealed at a normal Christmas season and many slopes were uncompromisingly hard ice which would normally · have been steep, firm snow; everyone in fact had to work fairly hard for the climbs they did. But some very good climbs were done. The existence (or should one say the ' invention ') of new ten thousanders must be a perpetual menace to the peace of mind of those few choice spirits who have achieved what they hoped was an unfading garland with the ascent of all the peaks in New Zealand over 1o,ooo ft. However, Mount ' Magellan ' is not positively claimed as definitely 1o,ooo ft., although it must closely approximate to it. (Throughout these notes names cited in inverted commas have yet to receive the approval of the Geographic Board.) ' Magellan ' lies on the Balfour Range spur of Mount Teichelmann, and its first ascent was made in February by Guide Harry Ayres with B. S. Gillies and Sir Edmund Hillary and D. G. Herron; the two parties climbed some of the day on one rope. They ieft the Haast Hut at 2 A.M., were· at the summit of Teichelmann by way of Clarke Saddle by 10.45 A.M., reached their objective, after traversing a long teetery crest of snow interrupted by a formidable rock tower, at 4 P.M., and returned to the Haast Hut by the route of ascent twenty-four hours after setting forth. -

Malaysia's Unique Biological Diversity: New Insights from Molecular Evolutionary Studies of Parasitic Flowering Plants
Malaysia's Unique Biological Diversity: New Insights from Molecular Evolutionary Studies of Parasitic Flowering Plants Daniel L. Nickrent Department of Plant Biology Southern Illinois University Carbondale, IL 62901-6509 e-mail: [email protected] October 2, 1995 ABSTRACT Malaysia is home to a rich assemblage of parasitic flowering plants representing seven families, 46 genera and approximately 100 species. These plants are seldom considered candidates for conservation efforts, however, many species are important components of the tropical ecosystem that show complex associations with other organisms and unique biochemical features. Results of a phylogenetic analysis of 29 members of Santalales using a combined dataset of nuclear-encoded 18S rDNA and plastid-encoded rbcL sequences are presented. Sequences from representatives of three nonphotosynthetic, holoparasitic families often allied with Santalales, Balanophoraceae, Hydnoraceae and Rafflesiaceae, have been obtained from nuclear-encoded 18S rDNA and plastid- encoded 16S rDNA. As with 18S rDNA, the 16S rDNA sequences from all three holoparasite families showed an increase in the number of substitutions. The greatest increases were seen in Mitrastema and Hydnora, greater than values obtained from pairwise comparisons involving taxa as phylogenetically distant as angiosperms and liverworts. A case is made that these plants represent unique natural genetic experiments that offer a wealth of opportunity for molecular genetic and phylogenetic analyses. 2 “We do not know enough about any gene, species, or ecosystem to be able to calculate its ecological and economic worth in the large scheme of things” (Ehrenfeld 1988) Why conserve parasitic plants? When considering the reasons for conservation of biodiversity, one inevitably concludes that all involve value judgments that are, in essence, anthropocentric. -

Wilderness Lodge Route Guide
Wilderness Lodge® Arthur’s Pass 16km East of Arthur’s Pass Village, Highway 73 [email protected] Wilderness Lodges +64 3318 9246 of New Zealand Wilderness Lodge® Lake Moeraki 90km South of Fox Glacier, Highway 6 wildernesslodge.co.nz [email protected] +64 3750 0881 Route Guide: Lake Moeraki to Arthur’s Pass This journey of 360km (about 200 miles) involves 5 to 6 hours of driving with great scenery and interesting stops along the way. We recom- mend that you allow as much time as possible. Key features include: beautiful rainforest; six large forested lakes; glistening snowy mountains and wild glacier rivers; the famous Fox and Franz Josef glaciers; the goldfields town of Hokitika; ascending Arthur’s Pass through the dramatic cleft of the Otira Gorge; and glorious alpine herbfields and shrublands at the summit. The times given below are driving times only. Enjoy Your Journey, Drive Safely & Remember to Keep Left Wilderness Lodge Lake Moeraki to Fox Glacier (92kms – 1¼ hrs) An easy drive through avenues of tall forest and lush farmland on mainly straight flat roads. Key features along this leg of the journey include Lake Paringa (20km), the Paringa River café and salmon farm (32km), a brief return to the coast at Bruce Bay (44km), and the crossing of three turbulent glacier rivers – the Karangarua (66km), Cook (86km) and Fox (90km) – at the point where they break free from the confines of their mountain valleys. In fair weather, striking views are available of the Sierra Range from the Karangarua River bridge (66km), Mt La Perouse (3079m) from the bridge across the Cook River (88km)and Mt Tasman (3498m) from the bridge over the Fox River (91km).The long summit ridge of Mt Cook (3754) is also briefly visible from just south of the Ohinetamatea River (15km north of the Karangarua River ) and again 4km further north on the approach to Bullock Creek. -

Chemical Weathering in Highsedimentyielding Watersheds
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110, F01008, doi:10.1029/2003JF000088, 2005 Chemical weathering in high-sediment-yielding watersheds, New Zealand W. Berry Lyons and Anne E. Carey Department of Geological Sciences and Byrd Polar Research Center, Ohio State University, Columbus, Ohio, USA D. Murray Hicks NIWA Research, Christchurch, New Zealand Carmen A. Nezat1 Byrd Polar Research Center, Ohio State University, Columbus, Ohio, USA Received 3 September 2003; revised 14 September 2004; accepted 30 November 2004; published 15 February 2005. [1] We have determined the chemical erosion yields for fifteen watersheds in New Zealand, ranging in size from 12.2 to 2928 km2. These rates, coupled with previously measured physical erosion yields, allow us to compare these two modes of landscape denudation. The physical erosion yields are some of the highest measured in the world. Although in most instances the chemical erosion yields are only a small fraction of the total erosion yields, the absolute values are very high. Our data strongly support the notion that chemical erosion rates are greatly influenced by the yield of physical erosion and that the rapid production of fresh surfaces as a result of high physical erosion rates and subsequent denudation is critical to the high chemical erosion yields observed. Citation: Lyons, W. B., A. E. Carey, D. M. Hicks, and C. A. Nezat (2005), Chemical weathering in high-sediment-yielding watersheds, New Zealand, J. Geophys. Res., 110, F01008, doi:10.1029/2003JF000088. 1. Introduction Vance et al., 2003]. In general, the net, large-scale erosional potential of a landscape is thought to increase with precip- [2] Over the past decade, a debate has occurred regard- itation, drainage area and slope [Montgomery et al., 2001]. -

Spatial Variation in Impacts of Brushtail Possums on Two Loranthaceous Mistletoe Species
SWEETAPPLE:Available on-line at:POSSUM http://www.newzealandecology.org/nzje/ IMPACTS ON MISTLETOE 177 Spatial variation in impacts of brushtail possums on two Loranthaceous mistletoe species Peter J. Sweetapple Landcare Research, PO Box 40, Lincoln 7640, New Zealand (Email: [email protected]) Published on-line: 8 October 2008 ___________________________________________________________________________________________________________________________________ Abstract: Browsing by introduced brushtail possums is linked to major declines in mistletoe abundance in New Zealand, yet in some areas mistletoes persist, apparently unaffected by the presence of possums. To determine the cause of this spatial variation in impact I investigated the abundance and condition (crown dieback and extent of possum browse cover) of two mistletoes (Alepis flavida, Peraxilla tetrapetala) and abundance and diet of possums in two mountain beech (Nothofagus solandri var. cliffortioides) forests in the central-eastern South Island of New Zealand. Mistletoe is common and there are long-established uncontrolled possum populations in both forests. Mistletoes were abundant (216–1359 per hectare) and important in possum diet (41–59% of total diet), but possum density was low (c. 2 per hectare) in both areas. Possum impacts were slight with low browse frequencies and intensities over much of the study sites. However, impacts were significantly greater at a forest margin, where possum abundance was highest, and at a high-altitude site where mistletoe density was lowest. Mistletoe crown dieback was inversely proportional to intensity of possum browsing. These results suggest that the persistence of abundant mistletoe populations at these sites is due to mistletoe productivity matching or exceeding consumption by possums in these forests of low possum-carrying _______________________________________________________________________________________________________________________________capacity, rather than low possum preference for the local mistletoe populations. -

Touring the West Coast - Ross to Hawea — NZ Walking Access Commission Ara Hīkoi Aotearoa
9/27/2021 Touring the West Coast - Ross to Hawea — NZ Walking Access Commission Ara Hīkoi Aotearoa Touring the West Coast - Ross to Hawea Difculty Hard Length 399.8 km Journey Time 6-8 days cycling Region West Coast Part of the Collection Nga Haerenga - The New Zealand Cycle Trail ROSS TO WHATAROA (75KM, 4–6 HOURS) From Ross, head south on Highway 6 for 5km and turn right down Bold Head Road. Then, head south on the highway to tranquil Lake Ianthe (26 km from Ross and good spot for a break). Continue down the highway to a small town called Harihari. About 75km from Ross is another small town called Whataroa, which is a good stopping place for the rst night. https://www.walkingaccess.govt.nz/track/touring-the-west-coast-ross-to-hawea/pdfPreview 1/5 9/27/2021 Touring the West Coast - Ross to Hawea — NZ Walking Access Commission Ara Hīkoi Aotearoa WHATAROA TO FOX GLACIER (53KM, 3–4 HOURS) Continue south, past Lake Wahapo and Lake Mapourika to Franz Josef township (30km from Whataroa). This tourist town has many services, and is a good base for a visit to the glacier. From Franz Josef township, the 23km journey south to Fox crosses three tough hills. Fox township is a similar size to Franz Josef. The DOC visitor centres at both towns are valuable sources of information. There are two excellent cycle paths leading to each glacier. Both are easy and about 10km long. They are signposted from just south of Franz Josef, and Fox townships.