Extant Earthly Microbial Mats and Microbialites As Models for Exploration of Life in Extraterrestrial Mat Worlds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and Their Association with the Endangered, Endemic Snail Physella Johnsoni
A Study on the Phototrophic Microbial Mat Communities of Sulphur Mountain Thermal Springs and their Association with the Endangered, Endemic Snail Physella johnsoni By Michael Bilyj A thesis submitted to the Faculty of Graduate Studies in partial fulfillment of the requirements for the degree of Master of Science Department of Microbiology Faculty of Science University of Manitoba Winnipeg, Manitoba October 2011 © Copyright 2011, Michael A. Bilyj 1 Abstract The seasonal population fluctuation of anoxygenic phototrophs and the diversity of cyanobacteria at the Sulphur Mountain thermal springs of Banff, Canada were investigated and compared to the drastic population changes of the endangered snail Physella johnsoni. A new species and two strains of Rhodomicrobium were taxonomically characterized in addition to new species of Rhodobacter and Erythromicrobium. Major mat-forming organisms included Thiothrix-like species, oxygenic phototrophs of genera Spirulina, Oscillatoria, and Phormidium and purple nonsulfur bacteria Rhodobacter, Rhodopseudomonas and Rhodomicrobium. Aerobic anoxygenic phototrophs comprised upwards of 9.6 x 104 CFU/cm2 of mat or 18.9% of total aerobic heterotrophic bacterial isolates at certain sites, while maximal purple nonsulfur and purple sulfur bacteria were quantified at 3.2 x 105 and 2.0 x 106 CFU/cm2 of mat, respectively. Photosynthetic activity measurements revealed incredibly productive carbon fixation rates averaging 40.5 mg C/cm2/24 h. A temporal mismatch was observed for mat area and prokaryote-based organics to P. johnsoni population flux in a ―tracking inertia‖ manner. 2 Acknowledgements It is difficult to express sufficient gratitude to my supervisor Dr. Vladimir Yurkov for his unfaltering patience, generosity and motivation throughout this entire degree. -

Thermophilic Lithotrophy and Phototrophy in an Intertidal, Iron-Rich, Geothermal Spring 2 3 Lewis M
bioRxiv preprint doi: https://doi.org/10.1101/428698; this version posted September 27, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Thermophilic Lithotrophy and Phototrophy in an Intertidal, Iron-rich, Geothermal Spring 2 3 Lewis M. Ward1,2,3*, Airi Idei4, Mayuko Nakagawa2,5, Yuichiro Ueno2,5,6, Woodward W. 4 Fischer3, Shawn E. McGlynn2* 5 6 1. Department of Earth and Planetary Sciences, Harvard University, Cambridge, MA 02138 USA 7 2. Earth-Life Science Institute, Tokyo Institute of Technology, Meguro, Tokyo, 152-8550, Japan 8 3. Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 9 91125 USA 10 4. Department of Biological Sciences, Tokyo Metropolitan University, Hachioji, Tokyo 192-0397, 11 Japan 12 5. Department of Earth and Planetary Sciences, Tokyo Institute of Technology, Meguro, Tokyo, 13 152-8551, Japan 14 6. Department of Subsurface Geobiological Analysis and Research, Japan Agency for Marine-Earth 15 Science and Technology, Natsushima-cho, Yokosuka 237-0061, Japan 16 Correspondence: [email protected] or [email protected] 17 18 Abstract 19 Hydrothermal systems, including terrestrial hot springs, contain diverse and systematic 20 arrays of geochemical conditions that vary over short spatial scales due to progressive interaction 21 between the reducing hydrothermal fluids, the oxygenated atmosphere, and in some cases 22 seawater. At Jinata Onsen, on Shikinejima Island, Japan, an intertidal, anoxic, iron- and 23 hydrogen-rich hot spring mixes with the oxygenated atmosphere and sulfate-rich seawater over 24 short spatial scales, creating an enormous range of redox environments over a distance ~10 m. -
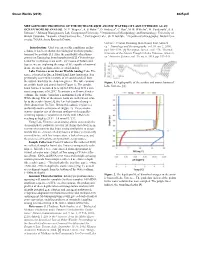
Metagenomic Profiling of the Methane-Rich Anoxic Waters of Lake Untersee As an Ocean Worlds Analog
Ocean Worlds (2019) 6025.pdf METAGENOMIC PROFILING OF THE METHANE-RICH ANOXIC WATERS OF LAKE UNTERSEE AS AN OCEAN WORLDS ANALOG. N. Y. Wagner1, A. S. Hahn2,3, D. Andersen4, C. Roy5, M. B. Wilhelm6, M. Vanderwilt1, S. S. Johnson1, 1 Johnson Biosignatures Lab, Georgetown University, 2 Department of Microbiology and Immunology, University of British Columbia, 3 Koonkie Cloud Services Inc., 4 Carl Sagan Center, SETI Institute, 5 Department of Geography, McGill Uni- versity, 6NASA Ames Research Center. Untersee, Central Dronning Maud Land, East Antarcti- Introduction: Under ocean worlds conditions on En- ca.” Limnology and Oceanography, vol. 51, no. 2, 2006, celadus, it has been shown that biological methane produc- pp.1180–1194, [4] Bevington, James, et al. “The Thermal tion may be possible [1]. Also, the possibility of methano- Structure of the Anoxic Trough in Lake Untersee, Antarcti- genesis on Europa has been hypothesized [2]. Given the po- ca.”Antarctic Science, vol. 30, no. 6, 2018, pp. 333–344. tential for methanogenesis on the icy moons of Saturn and Jupiter, we are exploring the range of life capable of survival in an extremely methane-rich terrestrial analog. Lake Untersee as an Ocean Worlds Analog: Lake Un- tersee is located in Queen Maud Land, East Antarctica. It is perennially covered in 3 meters of ice and closed off from the outside world by the Anuchin glacier. The lake contains Figure 1. Depth profile of the aerobic and anoxic basins of an aerobic basin and anoxic basin (Figure 1). The aerobic Lake Untersee [4]. basin has been measured to be up to 169m deep with a con- stant temperature of 0.25˚C. -

The Vulnerability of Microbial Ecosystems in a Changing Climate: Potential Impact in Shark Bay
life Review The Vulnerability of Microbial Ecosystems in a Changing Climate: Potential Impact in Shark Bay Max Reinold 1,2, Hon Lun Wong 1,2, Fraser I. MacLeod 1,2, Julia Meltzer 1,2, April Thompson 1,2 and Brendan P. Burns 1,2,* 1 School of Biotechnology and Biomolecular Sciences, The University of New South Wales, Sydney 2052, Australia 2 Australian Centre for Astrobiology, The University of New South Wales, Sydney 2052, Australia * Correspondence: [email protected]; Tel.: +612-9385-3659; Fax: +612-9385-1591 Received: 30 July 2019; Accepted: 28 August 2019; Published: 2 September 2019 Abstract: The potential impact of climate change on eukaryotes, including humans, has been relatively well described. In contrast, the contribution and susceptibility of microorganisms to a changing climate have, until recently, received relatively less attention. In this review, the importance of microorganisms in the climate change discourse is highlighted. Microorganisms are responsible for approximately half of all primary production on earth, support all forms of macroscopic life whether directly or indirectly, and often persist in “extreme” environments where most other life are excluded. In short, microorganisms are the life support system of the biosphere and therefore must be included in decision making regarding climate change. Any effects climate change will have on microorganisms will inevitably impact higher eukaryotes and the activity of microbial communities in turn can contribute to or alleviate the severity of the changing climate. It is of vital importance that unique, fragile, microbial ecosystems are a focus of research efforts so that their resilience to extreme weather events and climate change are thoroughly understood and that conservation efforts can be implemented as a response. -

Stromatolites
Stromatolites What is a stromatolite? A stromatolite (literally, ‘layered rock’) is a solid structure created by single-celled microbes called cyanobacteria (blue-green algae). The cyanobacteria form colonies and trap sediment with their sticky surface coatings. The trapped sediment reacts to calcium carbonate in the water to form limestone. These limestone deposits build up very slowly – it can take a stromatolite 100 years to grow 5 cm. A 1 m-high stromatolite might be 2,000 years old! Where are they found? Shark Bay’s stromatolites are found around the shallows of Hamelin Pool , located in the southern part of the eastern bay. Between 4,000 to 6,000 years ago a massive seagrass bank called the Fauré Sill began to block tidal flow into Hamelin Pool, causing the water to become extremely concentrated, or hypersaline. The water in Hamelin Pool is twice as salty as water in the open ocean! Animals that would normally graze on algae, such as chitons and snails, cannot survive in these conditions. Around 3,000 years ago cyanobacteria started flourishing, forming stromatolites much as they did billions of years ago. More than 50 species of cyanobacteria live in Hamelin Pool. What do they look like? Stromatolites look like a cross between a cauliflower and a rock. However, unlike rocks they are actually alive – each stromatolite has a top surface layer teeming with living, active cyanobacteria. At least 3,000 million cyanobacteria can fit in 1 m2! Because cyanobacteria are plants, they photosynthesise their energy from the sun. A by-product of photosynthesis is oxygen, and if you look very carefully you may see the stromatolites gently ‘fizzing’ as tiny bubbles of oxygen are released by the cyanobacteria into the water. -

Stone Aerospace Saves 12 Weeks in Cabling Design by Implementing a Digital Design Process
SUCCESS STORY ® Zuken’s software solution for electrical wiring, control systems and fluid engineering. Stone Aerospace saves 12 weeks in cabling design by implementing a digital design process “We achieved substantial productivity gains because engineers had a very clear picture of what one another were doing, and because of the automated checks performed by the software.“ John Harman, Electrical Engineer, Stone Aerospace ZUKEN - The Partner for Success SUCCESS STORY Stone Aerospace saves 12 weeks in cabling design by implementing a digital design process Stone Aerospace faced the pressure of a tight schedule in designing Results a one-of-a-kind underwater autonomous vehicle (AUV) capable • Elimination of $20,000 in cable of traveling 15km under the Antarctic ice shelf. The AUV acts as a rework and expedited delivery costs testing ground to validate an aircraft-mounted radar system that • Design cycle reduction by 12 weeks will be used in a space mission. The time needed to design the wiring • Ability to view the electrical and harness for the AUV was reduced by around 12 weeks and $20,000 physical design of the entire craft in was saved by using E3.series to automate many aspects of the design a single hierarchical view process, while integrating the logical and physical design on a single • E ective design team collaboration platform. created common nomenclature improving quality and cutting errors The search for life in space aspects such as temperature, depth and water current velocities, and to identify • Automated checks ensured correct Scientists believe that underneath the microbiological communities. connector selections. icy surface of Europa, one of Jupiter’s moons, is a vast ocean thought to be the Cable design challenges most likely location for finding life in our solar system. -

Algal Toxic Compounds and Their Aeroterrestrial, Airborne and Other Extremophilic Producers with Attention to Soil and Plant Contamination: a Review
toxins Review Algal Toxic Compounds and Their Aeroterrestrial, Airborne and other Extremophilic Producers with Attention to Soil and Plant Contamination: A Review Georg G¨аrtner 1, Maya Stoyneva-G¨аrtner 2 and Blagoy Uzunov 2,* 1 Institut für Botanik der Universität Innsbruck, Sternwartestrasse 15, 6020 Innsbruck, Austria; [email protected] 2 Department of Botany, Faculty of Biology, Sofia University “St. Kliment Ohridski”, 8 blvd. Dragan Tsankov, 1164 Sofia, Bulgaria; mstoyneva@uni-sofia.bg * Correspondence: buzunov@uni-sofia.bg Abstract: The review summarizes the available knowledge on toxins and their producers from rather disparate algal assemblages of aeroterrestrial, airborne and other versatile extreme environments (hot springs, deserts, ice, snow, caves, etc.) and on phycotoxins as contaminants of emergent concern in soil and plants. There is a growing body of evidence that algal toxins and their producers occur in all general types of extreme habitats, and cyanobacteria/cyanoprokaryotes dominate in most of them. Altogether, 55 toxigenic algal genera (47 cyanoprokaryotes) were enlisted, and our analysis showed that besides the “standard” toxins, routinely known from different waterbodies (microcystins, nodularins, anatoxins, saxitoxins, cylindrospermopsins, BMAA, etc.), they can produce some specific toxic compounds. Whether the toxic biomolecules are related with the harsh conditions on which algae have to thrive and what is their functional role may be answered by future studies. Therefore, we outline the gaps in knowledge and provide ideas for further research, considering, from one side, Citation: G¨аrtner, G.; the health risk from phycotoxins on the background of the global warming and eutrophication and, ¨а Stoyneva-G rtner, M.; Uzunov, B. -

(Antarctica) Glacial, Basal, and Accretion Ice
CHARACTERIZATION OF ORGANISMS IN VOSTOK (ANTARCTICA) GLACIAL, BASAL, AND ACCRETION ICE Colby J. Gura A Thesis Submitted to the Graduate College of Bowling Green State University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE December 2019 Committee: Scott O. Rogers, Advisor Helen Michaels Paul Morris © 2019 Colby Gura All Rights Reserved iii ABSTRACT Scott O. Rogers, Advisor Chapter 1: Lake Vostok is named for the nearby Vostok Station located at 78°28’S, 106°48’E and at an elevation of 3,488 m. The lake is covered by a glacier that is approximately 4 km thick and comprised of 4 different types of ice: meteoric, basal, type 1 accretion ice, and type 2 accretion ice. Six samples were derived from the glacial, basal, and accretion ice of the 5G ice core (depths of 2,149 m; 3,501 m; 3,520 m; 3,540 m; 3,569 m; and 3,585 m) and prepared through several processes. The RNA and DNA were extracted from ultracentrifugally concentrated meltwater samples. From the extracted RNA, cDNA was synthesized so the samples could be further manipulated. Both the cDNA and the DNA were amplified through polymerase chain reaction. Ion Torrent primers were attached to the DNA and cDNA and then prepared to be sequenced. Following sequencing the sequences were analyzed using BLAST. Python and Biopython were then used to collect more data and organize the data for manual curation and analysis. Chapter 2: As a result of the glacier and its geographic location, Lake Vostok is an extreme and unique environment that is often compared to Jupiter’s ice-covered moon, Europa. -

Carbon Cycling and Calcification in Hypersaline Microbial Mats
Carbon cycling and calcification in hypersaline microbial mats Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften - Dr. rer. nat.- Dem Fachbereich Biologie/Chemie der Universität Bremen vorgelegt von Rebecca Ludwig Bremen März 2004 Die vorliegende Arbeit wurde in der Zeit von Oktober 2000 bis März 2004 am Max-Planck-Institut für marine Mikrobiologie in Bremen angefertigt. Gutachter Prof. Dr. Bo Barker Jørgensen Prof. Dr. Gunter O. Kirst Prüfer Prof. Dr. Friederike Koenig Dr. Henk M. Jonkers Tag des Promotionskolloqiums: 14. Mai 2004 Table of contents Table of contents Thesis outline v 1 Introduction 1 Prologue 3 Carbon cycle in microbial mats: Organisms and metabolism 8 Gradients and adaptations to diel changes 17 Calcification 20 Principles and applications of microsensors 22 Sampling/study sites 28 2 Structure and function of Chiprana mats 37 Structural and functional analysis of a microbial mat ecosystem from a unique permanent hypersaline inland lake: ‘La Salada de Chiprana’ (NE Spain) 3 Rate limitation in microbial mats 71 Limitation of oxygenic photosynthesis and respiration by phosphate and organic nitrogen in a hypersaline mat: A microsensor study 4 Effect of salinity on benthic photosynthesis 89 Reduced gas diffusivity and solubility limit metabolic rates in benthic phototrophs at high salinities 5 Calcification mechanism in a microbial mat 109 Photosynthesis controlled calcification in a hypersaline microbial mat 6 Stromatolite calcification and bioerosion 127 Balance between microbial calcification and metazoan bioerosion in modern stromatolitic oncolites Discussion 145 Summary 151 Zusammenfassung 153 Appendix 155 Danksagung 155 List of publications 157 iii Outline Thesis outline Five manuscripts are included in this thesis that investigate the carbon cycle in microbial mats with a special emphasis on community carbon flow and mechanisms of microbial calcification. -

Subglacial Lake Ellsworth CEE V2.Indd
Proposed Exploration of Subglacial Lake Ellsworth Antarctica Draft Comprehensive Environmental Evaluation February 2011 1 The cover art was created by first, second, and third grade students at The Village School, a Montessori school located in Waldwick, New Jersey. Art instructor, Bob Fontaine asked his students to create an interpretation of the work being done on The Lake Ellsworth Project and to create over 200 of their own imaginary microbes. This art project was part of The Village School’s art curriculum that links art with ongoing cultural work. 2 Contents Non Technical Summary .................................................4 Personnel ............................................................................................ 26 Chapter 1: Introduction ...................................................6 Power generation and fuel calculations ....................................... 27 Chapter 2: Description of proposed activity..................7 Vehicles ................................................................................................ 28 Background and justification ..............................................................7 Water and waste ...............................................................................28 The site ...................................................................................................8 Communications ............................................................................... 28 Chapter 3: Baseline conditions of Lake Ellsworth .........9 Transport of equipment -

Subglacial Meltwater Supported Aerobic Marine Habitats During Snowball Earth
Subglacial meltwater supported aerobic marine habitats during Snowball Earth Maxwell A. Lechtea,b,1, Malcolm W. Wallacea, Ashleigh van Smeerdijk Hooda, Weiqiang Lic, Ganqing Jiangd, Galen P. Halversonb, Dan Asaele, Stephanie L. McColla, and Noah J. Planavskye aSchool of Earth Sciences, University of Melbourne, Parkville, VIC 3010, Australia; bDepartment of Earth and Planetary Science, McGill University, Montréal, QC, Canada H3A 0E8; cState Key Laboratory for Mineral Deposits Research, School of Earth Sciences and Engineering, Nanjing University, 210093 Nanjing, China; dDepartment of Geoscience, University of Nevada, Las Vegas, NV 89154; and eDepartment of Geology and Geophysics, Yale University, New Haven, CT 06511 Edited by Paul F. Hoffman, University of Victoria, Victoria, BC, Canada, and approved November 3, 2019 (received for review May 28, 2019) The Earth’s most severe ice ages interrupted a crucial interval in Cryogenian ice age. These marine chemical sediments are unique eukaryotic evolution with widespread ice coverage during the geochemical archives of synglacial ocean chemistry. To develop Cryogenian Period (720 to 635 Ma). Aerobic eukaryotes must have sur- a global picture of seawater redox state during extreme glaci- vived the “Snowball Earth” glaciations, requiring the persistence of ation, we studied 9 IF-bearing Sturtian glacial successions across 3 oxygenated marine habitats, yet evidence for these environments paleocontinents (Fig. 1): Congo (Chuos Formation, Namibia), is lacking. We examine iron formations within globally distributed Australia (Yudnamutana Subgroup), and Laurentia (Kingston Cryogenian glacial successions to reconstruct the redox state of the Peak Formation, United States). These IFs were selected for synglacial oceans. Iron isotope ratios and cerium anomalies from a analysis because they are well-preserved, and their depositional range of glaciomarine environments reveal pervasive anoxia in the environment can be reliably constrained. -

Development of Nereid-UI: a Remotely Operated Underwater Vehicle for Oceanographic Access Under Ice
Development of Nereid-UI: A Remotely Operated Underwater Vehicle for Oceanographic Access Under Ice Presented at the 9th Annual Polar Technology Conference, 2-4 April 2013 Louis L. Whitcomb1,2,Andrew D. Bowen1, Dana R. Yoerger1, Christopher German1, James C. Kinsey1, Larry Mayer1,3, Michael V. Jakuba1, Daniel Gomez-Ibanez1, Christopher L. Taylor1, Casey Machado1, Jonathan C. Howland1, Carl Kaiser1, Matthew Heintz1 1Department of Applied Ocean Physics and Engineering, Woods Hole Oceanographic Institution 2Department of Mechanical Engineering, Johns Hopkins University 3Center for Coastal and Ocean Mapping, University of New Hampshire Photo courtesy S. McPhail, NOC Woods Hole Oceanographic Institution World’s largest private ocean research institution ~900 Employees, 143 Scientific Staff $160M Annual Budget • Biology • Chemistry • Geology • Physical Oceanography • Engineering • Marine Policy Deep Ocean Oceanography: The D.S.V. Alvin 4500m Submersible Image Credit: Rod Catenach © WHOI Catenach Credit: Rod Image Crew: 3 = 1 pilot + 2 scientist Depth: 4500m (6,500m soon) Endurance: 6-10 Hours Speed: 1 m/s Mass: 7,000 Kg Length: 7.1m Power: 81 KWH Life Support: 72 Hours x 3 Persons Ph.D. Student James Kinsey Dives: >4,700 (since 1964) Passengers: >14,000 (since 1964) Jason II ROV Specifications: Size: 3.2 x 2.4 x 2.2 m Weight 3,300 kg Depth 6,500 m Power 40 kW (50 Hp) Payload: 120 Kg (1.5 Ton) First Dive: 2002 Dives: >600 Dive Time: >12,500 Hours* Bottom Time: >10,600 Hours* Longest Dive: 139 Hours* Deepest Dive: 6,502 m* Distance: >4,800 km* * As of Feb, 2012 Electric thrusters, twin hydraulic manipulator arms.