Dynavax Announces Second Quarter 2021 Financial Results
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Valneva Initiates Phase 3 Clinical Trial for Its Inactivated, Adjuvanted COVID-19 Vaccine Candidate, VLA2001
VALNEVA SE Campus Bio-Ouest | 6, Rue Alain Bombard 44800 Saint-Herblain, France Valneva Initiates Phase 3 Clinical Trial for its Inactivated, Adjuvanted COVID-19 Vaccine Candidate, VLA2001 Saint-Herblain (France), April 21, 2021 – Valneva SE, a specialty vaccine company focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need, today announced it has initiated a pivotal Phase 3 clinical trial for its inactivated, adjuvanted COVID-19 vaccine candidate, VLA2001. The Phase 3 trial “Cov-Compare”, (VLA2001-301), will compare Valneva’s SARS-CoV-2 vaccine candidate, VLA2001, against AstraZeneca’s conditionally approved vaccine, Vaxzevria1, in a comparative immunogenicity trial. Approximately 4,000 participants will receive two doses of either vaccine. The primary endpoint of Cov- Compare will be to determine the immune response (Geometric Mean Titer (GMT)) of SARS-CoV-2- specific neutralizing antibodies) two weeks after completion of a two-dose immunization schedule administered in a four-week interval. The trial is powered to demonstrate superiority of VLA2001 in terms of GMT ratio (VLA2001/Vaxzevria). The trial will be conducted in the U.K. and is supported by the National Institute for Health Research (NIHR). Adam Finn, Chief investigator for the VLA 2001-301 program, Professor of Paediatrics at the University of Bristol and Consultant at the Bristol Royal Hospital for Children said, “Following very encouraging safety and immune response results from our Phase 1/2 trial, along with my investigator colleagues, I am really looking forward to starting on this important next stage of the clinical development of this important new vaccine. -

Valneva Commences Rolling Submission to MHRA for Its Inactivated, Adjuvanted COVID-19 Vaccine
VALNEVA SE Campus Bio-Ouest | 6, Rue Alain Bombard 44800 Saint-Herblain, France Valneva Commences Rolling Submission to MHRA for its Inactivated, Adjuvanted COVID-19 Vaccine Saint-Herblain (France), August 23, 2021 – Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company focused on prophylactic vaccines for infectious diseases, today commenced rolling submission, for initial approval of its COVID-19 vaccine candidate, VLA2001, with the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom (UK). VLA2001 is a whole virus, inactivated, adjuvanted vaccine candidate and is the only vaccine candidate of this type currently in clinical trials in Europe. VLA2001 is currently being studied in the UK in a pivotal Phase 3 trial, “Cov-Compare” (VLA2001-301), for which topline results are currently expected early in the fourth quarter 2021. Subject to positive Cov- Compare data and MHRA review, Valneva believes that initial approval could be granted before the end of 2021. In September 2020, Valneva announced a collaboration with the UK Government, which has the option to purchase up to 190 million doses through 2025. So far, the UK Government has ordered 100 million doses for supply in 2021 and 2022. Juan Carlos Jaramillo M.D., Chief Medical Officer of Valneva, said, “We are pleased to begin the regulatory review process for our COVID-19 vaccine with the MHRA. Valneva believes that everyone should have access to technology best suited to protect them against this virus. We are working hard to make our vaccine candidate available as soon as possible. We are grateful to the National Institute for Health Research (NIHR), Public Health England (PHE), and other partners for their unstinting support and hard work.” Valneva recently initiated a further Phase 3 clinical trial, VLA2001-304, to generate data in the elderly and as part of the Company’s strategy to evaluate variant-based vaccines. -
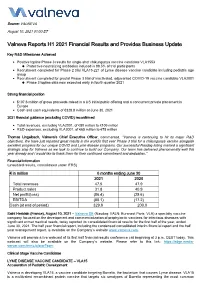
Valneva Reports H1 2021 Financial Results and Provides Business Update
Source: VALNEVA August 10, 2021 01:00 ET Valneva Reports H1 2021 Financial Results and Provides Business Update Key R&D Milestones Achieved Positive topline Phase 3 results for single-shot chikungunya vaccine candidate VLA1553 Protective neutralizing antibodies induced in 98.5% of trial participants Recruitment completed for Phase 2 trial VLA15-221 of Lyme disease vaccine candidate including pediatric age group Recruitment completed for pivotal Phase 3 trial of inactivated, adjuvanted COVID-19 vaccine candidate VLA2001 Phase 3 topline data now expected early in fourth quarter 2021 Strong financial position $107.6 million of gross proceeds raised in a US initial public offering and a concurrent private placement in Europe Cash and cash equivalents of €329.8 million at June 30, 2021 2021 financial guidance (excluding COVID) reconfirmed Total revenues, excluding VLA2001, of €80 million to €105 million R&D expenses, excluding VLA2001, of €65 million to €75 million Thomas Lingelbach, Valneva’s Chief Executive Officer, commented, “Valneva is continuing to hit its major R&D objectives. We have just reported great results in the world’s first ever Phase 3 trial for a chikungunya vaccine alongside excellent progress for our unique COVID and Lyme disease programs. Our successful Nasdaq listing marked a significant strategic step for Valneva as we look to continue to build our Company. Our team has delivered phenomenally well this year already and I would like to thank them for their continued commitment and dedication.” Financial Information (unaudited -

Press Release
VALNEVA SE Campus Bio-Ouest | 6, Rue Alain Bombard 44800 Saint-Herblain, France Valneva to Participate in the World’s First COVID-19 Vaccine Booster Trial in the UK Saint-Herblain (France), May 19, 2021 – Valneva SE, a specialty vaccine company focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need, today announced it will participate in a UK government-funded clinical trial looking at different COVID-19 ‘booster’ vaccines that launches today. The Cov-Boost trial, led by University Hospital Southampton NHS Foundation Trust, will look at seven different COVID-19 vaccines, including Valneva’s inactivated vaccine VLA2001, as potential boosters. It will be the first trial in the world to provide vital data on how effective a booster of each vaccine is in protecting individuals from the virus. The vaccines will be given at least three months after a second dose as part of the ongoing vaccination programme. One booster will be provided to each volunteer and could be a different brand to the one they were originally vaccinated with. The trial will also include a control group. Initial results from the trial, expected in September, will help inform decisions by the UK Joint Committee on Vaccination and Immunisation (JCVI) on any potential booster programme from autumn this year, ensuring the UK’s most vulnerable people are given the strongest possible protection over the winter period. Thomas Lingelbach, Chief Executive Officer of Valneva, commented, “We are pleased to be involved in this new Cov-Boost trial. Valneva has the only whole virus, inactivated, adjuvanted vaccine candidate in clinical trials against COVID-19 in Europe and we believe our VLA2001 vaccine has an important role to play in the ongoing pandemic, including as a booster. -

Valneva Reports Positive Phase 1/2 Data for Its Inactivated, Adjuvanted COVID-19 Vaccine Candidate, VLA2001
VALNEVA SE Campus Bio-Ouest | 6, Rue Alain Bombard 44800 Saint-Herblain, France Valneva Reports Positive Phase 1/2 Data for Its Inactivated, Adjuvanted COVID-19 Vaccine Candidate, VLA2001 VLA2001 was well tolerated with no safety concerns identified In the high dose group : - IgG seroconversion rate of 100% - Neutralizing antibody titres at or above levels generally seen in convalescent sera. Saint-Herblain (France), April 6, 2021 – Valneva SE, a specialty vaccine company focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need, today announced positive data for Part A of the Phase 1/2 clinical trial of its inactivated, adjuvanted COVID-19 vaccine candidate, VLA2001. Based on this data, the Company plans to commence a Phase 3 clinical trial by the end of April 2021, subject to regulatory approval. In study VLA2001-201, three dose levels of VLA2001 (low, medium, high), based on a schedule of two doses with vaccinations three weeks apart, were evaluated in 153 healthy adults aged 18 to 55 years. VLA2001 was generally well tolerated across all dose groups tested, with no safety concerns identified by an independent Data Safety Monitoring Board. There were no statistically significant differences between dose groups and no differences between first and second vaccinations in terms of reactogenicity. The majority of Adverse Events (AEs) were mild or moderate and only two subjects reported severe solicited AEs (headache and fatigue). All solicited AEs were transient. Only 17.6% of unsolicited AEs up to day 36 were considered related to the vaccine and no severe unsolicited AEs were reported. -

Valneva Reports FY 2020 Results and Major Corporate Achievements
VALNEVA SE Campus Bio-Ouest | 6, Rue Alain Bombard 44800 Saint-Herblain, France Valneva Reports FY 2020 Results and Major Corporate Achievements Excellent progress on clinical programs o Unprecedented partnering deal signed with Pfizer for Lyme disease vaccine candidate VLA15, including $130 million upfront payment as part of over $300 million for upfront and milestone payments . Positive initial Phase 2 results . Acceleration of pediatric development announced o Acceleration of chikungunya vaccine candidate VLA1553 into Phase 3 . Only Phase 3 chikungunya vaccine program to date worldwide . Potentially eligible for Priority Review Voucher (PRV)1 - for first company to receive Biologics License Application (BLA) approval Commercial business adversely affected by pandemic impact on travel industry o New US military / Department of Defense (DoD) contract for IXIARO® worth up to $166 million over three years Valneva contributing to the global effort against the COVID-19 pandemic o Only whole-virus inactivated vaccine candidate in clinical development in Europe . Commercial manufacturing commenced o Major Partnership with U.K. government, worth up to €1.4 billion including investment in manufacturing plant and clinical trials in the UK . Advanced discussions with European Commission FY 2020 cash and cash equivalents of €204.4 million underlining strong balance sheet EGM passes resolutions to allow preparation for possible Nasdaq listing and US IPO Thomas Lingelbach, Chief Executive Officer, commented, “2020 was a transformational year for Valneva, marked by major partnerships with Pfizer and the UK government as well as substantial progress across all of our clinical programs. Our company has responded very well to the challenges presented to us by the COVID pandemic. -
Valneva SE Form 6-K Current Event Report Filed 2021-08-23
SECURITIES AND EXCHANGE COMMISSION FORM 6-K Current report of foreign issuer pursuant to Rules 13a-16 and 15d-16 Amendments Filing Date: 2021-08-23 | Period of Report: 2021-08-23 SEC Accession No. 0001171843-21-006111 (HTML Version on secdatabase.com) FILER Valneva SE Mailing Address Business Address 6, RUE ALAIN BOMBARD 6, RUE ALAIN BOMBARD CIK:1836564| IRS No.: 000000000 | State of Incorp.:I0 | Fiscal Year End: 1231 SAINT-HERBLAIN I0 44800 SAINT-HERBLAIN I0 44800 Type: 6-K | Act: 34 | File No.: 001-40377 | Film No.: 211197819 33 2 28 07 37 10 SIC: 2836 Biological products, (no disgnostic substances) Copyright © 2021 www.secdatabase.com. All Rights Reserved. Please Consider the Environment Before Printing This Document UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 6-K REPORT OF FOREIGN PRIVATE ISSUER PURSUANT TO RULE 13a-16 OR 15d-16 UNDER THE SECURITIES EXCHANGE ACT OF 1934 Date of Report: August 23, 2021 Commission File Number: 001-40377 Valneva SE (Translation of registrant's name into English) 6 rue Alain Bombard 44800 Saint-Herblain, France (Address of principal executive office) Indicate by check mark whether the registrant files or will file annual reports under cover of Form 20-F or Form 40-F. Form 20-F [ X ] Form 40-F [ ] Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1): Note: Regulation S-T Rule 101(b)(1) only permits the submission in paper of a Form 6-K if submitted solely to provide an attached annual report to security holders. -

Valneva to Host Symposium on COVID-19 and Chikungunya Vaccine Candidates at 31St European Congress of Clinical Microbiology &
VALNEVA SE Campus Bio-Ouest | 6, Rue Alain Bombard 44800 Saint-Herblain, France Valneva to Host Symposium on COVID-19 and Chikungunya Vaccine Candidates at 31st European Congress of Clinical Microbiology & Infectious Diseases Saint Herblain (France), July 5, 2021 –Valneva SE (Nasdaq: VALN; Euronext Paris: VLA), a specialty vaccine company focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need, announced today it will host a virtual symposium titled, “Developing new vaccines to protect against infectious diseases at home and abroad” on July 9, 2021 at 14:15 CEST at the 31st European Congress of Clinical Microbiology & Infectious Diseases (ECCMID). The symposium will be chaired by Prof. Thea Kølsen Fischer, Danish epidemiologist and member of the WHO team investigating the origins of SARS-CoV-2, and Katrin Dubischar, VP Program Director Chikungunya Vaccine at Valneva. Adam Finn, Principal investigator for Valneva’s COVID-19 program, Professor of Paediatrics at the University of Bristol and Consultant at the Bristol Royal Hospital for Children, will discuss Phase 1/2 data of VLA2001, the only whole-virus, inactivated adjuvanted COVID-19 vaccine candidate in clinical trials in Europe. Prof. Thomas Jelinek, renowned key opinion leader and Medical Director of Berlin Centre for Travel and Tropical Medicine, will present on chikungunya disease and results of the Phase 1 study of Valneva’s single-shot chikungunya vaccine candidate VLA1553. To attend the ECCMID conference and participate in Valneva’s symposium, you can register here. About VLA2001 VLA2001 is currently the only whole virus, inactivated, adjuvanted vaccine candidate in clinical trials against COVID-19 in Europe. -
Valneva Reports H1 2021 Financial Results and Provides Business Update
VALNEVA SE Campus Bio-Ouest | 6, Rue Alain Bombard 44800 Saint-Herblain, France Valneva Reports H1 2021 Financial Results and Provides Business Update Key R&D Milestones Achieved Positive topline Phase 3 results for single-shot chikungunya vaccine candidate VLA1553 o Protective neutralizing antibodies induced in 98.5% of trial participants Recruitment completed for Phase 2 trial VLA15-221 of Lyme disease vaccine candidate including pediatric age group Recruitment completed for pivotal Phase 3 trial of inactivated, adjuvanted COVID-19 vaccine candidate VLA2001 o Phase 3 topline data now expected early in fourth quarter 2021 Strong financial position $107.6 million of gross proceeds raised in a US initial public offering and a concurrent private placement in Europe Cash and cash equivalents of €329.8 million at June 30, 2021 2021 financial guidance (excluding COVID) reconfirmed Total revenues, excluding VLA2001, of €80 million to €105 million R&D expenses, excluding VLA2001, of €65 million to €75 million Thomas Lingelbach, Valneva’s Chief Executive Officer, commented, “Valneva is continuing to hit its major R&D objectives. We have just reported great results in the world’s first ever Phase 3 trial for a chikungunya vaccine alongside excellent progress for our unique COVID and Lyme disease programs. Our successful Nasdaq listing marked a significant strategic step for Valneva as we look to continue to build our Company. Our team has delivered phenomenally well this year already and I would like to thank them for their continued commitment -

Valneva Initiates Phase 1/2 Clinical Study of Inactivated, Adjuvanted COVID-19 Vaccine Candidate – Valneva
12/20/2020 Valneva Initiates Phase 1/2 Clinical Study of Inactivated, Adjuvanted COVID-19 Vaccine Candidate – Valneva About Us Products R&D Investors Media Careers CONTACT EN | FR Valneva Initiates Phase 1/2 Clinical Study of Inactivated, Adjuvanted COVID-19 Vaccine Candidate December 16, 2020 Saint-Herblain (France), December 16, 2020 – Valneva SE, a specialty vaccine company focused on prevention against diseases with major unmet needs, today announced the initiation of a Phase 1/2 clinical study for its inactivated, adjuvanted COVID-19 vaccine candidate, VLA2001. VLA2001 leverages the manufacturing platform of Valneva’s licensed Japanese encephalitis vaccine, IXIARO® and is the first publicly announced inactivated vaccine against COVID-19 to commence clinical development in Europe. The VLA2001-201 study is a randomized, double blind trial evaluating the safety and immunogenicity for three dose levels in approximately 150 healthy adults. The study will be conducted in study sites across the United Kingdom and is supported by the National Institute for Health Research (NIHR). The primary endpoint read-out will be two weeks after completion of the two-dose primary immunization (day 0, 21). Subject to analysis of this data, including the https://valneva.com/press-release/valneva-initiates-phase-1-2-clinical-study-of-inactivated-adjuvanted-covid-19-vaccine-candidate/ 1/5 12/20/2020 Valneva Initiates Phase 1/2 Clinical Study of Inactivated, Adjuvanted COVID-19 Vaccine Candidate – Valneva selection of the optimal dose currently expected in the early second quarter of 2021, Abaodudt itUiosnal tPrriaolds uacrtes expecR&tDed toI ncvoesmmtoersnce iMmedmeiadiateClya rteerherseafter. CONTACT EN | FR The Company currently plans to include more than 4,000 participants in additional trials, which it believes could support an initial regulatory approval as soon as the fourth quarter of 2021. -

Valneva Reports H1 2021 Financial Results and Provides Business Update
VALNEVA SE Campus Bio-Ouest | 6, Rue Alain Bombard 44800 Saint-Herblain, France Valneva Reports H1 2021 Financial Results and Provides Business Update Key R&D Milestones Achieved Positive topline Phase 3 results for single-shot chikungunya vaccine candidate VLA1553 o Protective neutralizing antibodies induced in 98.5% of trial participants Recruitment completed for Phase 2 trial VLA15-221 of Lyme disease vaccine candidate including pediatric age group Recruitment completed for pivotal Phase 3 trial of inactivated, adjuvanted COVID-19 vaccine candidate VLA2001 o Phase 3 topline data now expected early in fourth quarter 2021 Strong financial position $107.6 million of gross proceeds raised in a US initial public offering and a concurrent private placement in Europe Cash and cash equivalents of €329.8 million at June 30, 2021 2021 financial guidance (excluding COVID) reconfirmed Total revenues, excluding VLA2001, of €80 million to €105 million R&D expenses, excluding VLA2001, of €65 million to €75 million Thomas Lingelbach, Valneva’s Chief Executive Officer, commented, “Valneva is continuing to hit its major R&D objectives. We have just reported great results in the world’s first ever Phase 3 trial for a chikungunya vaccine alongside excellent progress for our unique COVID and Lyme disease programs. Our successful Nasdaq listing marked a significant strategic step for Valneva as we look to continue to build our Company. Our team has delivered phenomenally well this year already and I would like to thank them for their continued commitment -

Valneva Completes Phase 3 Trial Recruitment for Its Inactivated COVID-19 Vaccine Candidate
VALNEVA SE Campus Bio-Ouest | 6, Rue Alain Bombard 44800 Saint-Herblain, France Valneva Completes Phase 3 Trial Recruitment for its Inactivated COVID-19 Vaccine Candidate Saint-Herblain (France), June 3, 2021 –Valneva SE, a specialty vaccine company focused on the development and commercialization of prophylactic vaccines for infectious diseases with significant unmet medical need, today announced that it has completed recruitment for the pivotal Phase 3 trial of its inactivated, adjuvanted COVID-19 vaccine candidate, VLA2001. Over 4,000 volunteers in the United Kingdom have been randomized in the Phase 3 trial “Cov- Compare” (VLA2001-301), which compares Valneva’s SARS-CoV-2 vaccine candidate, VLA2001, against AstraZeneca’s conditionally approved vaccine, Vaxzevria1. Cov-Compare’s primary endpoint is to determine the immune response (Geometric Mean Titer – GMT of SARS-CoV-2- specific neutralizing antibodies) two weeks after completion of a two-dose immunization schedule administered in a four-week interval. Topline data are expected by September 2021 and submission to the UK’s Medicines and Healthcare products Regulatory Agency for regulatory approval will follow, subject to the topline data. Juan Carlos Jaramillo, M.D., Chief Medical Officer of Valneva, commented, “We are extremely pleased to have achieved this important milestone in such a short period of time. I would like to thank the UK Vaccines Taskforce, the National Institute for Health Research and trial sites who have played vital roles in the rapid recruitment and enrollment of volunteers for the clinical trial. Based on our Phase 1/2 clinical data and, assuming successful Phase 3 results, we believe that our inactivated vaccine can make a major contribution to the ongoing fight against the COVID-19 pandemic.” UK Health and Social Care Secretary, Matt Hancock, said, “The UK government has fully supported this promising COVID-19 vaccine by funding the early stage clinical trials and helping to recruit patients through the National Institute for Health Research.