Freshwater Origin of Primary Plastids COMMENTARY Louise A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Origin of Gibberellin-Dependent Transcriptional Regulation by Molecular Exploitation of a Transactivation Domain in DELLA Proteins
bioRxiv preprint doi: https://doi.org/10.1101/398883; this version posted December 10, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Article - Discoveries Origin of gibberellin-dependent transcriptional regulation by molecular exploitation of a transactivation domain in DELLA proteins Jorge Hernández-García1, Asier Briones-Moreno1, Renaud Dumas2, and Miguel A. Blázquez1 1Instituto de Biología Molecular y Celular de Plantas (IBMCP), CSIC-Universidad Politécnica de Valencia, Campus UPV CPI 8E, Valencia, Spain 2Laboratoire de Physiologie Cellulaire et Végétale, Université Grenoble Alpes, CNRS, Commissariat à l'Energie Atomique et aux Energies Alternatives/Biosciences and Biotechnology Institute of Grenoble, Institut National de la Recherche Agronomique (INRA), Grenoble, France Corresponding author: Miguel A. Blázquez ([email protected]) 1 bioRxiv preprint doi: https://doi.org/10.1101/398883; this version posted December 10, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Abstract DELLA proteins are land-plant specific transcriptional regulators known to interact through their C-terminal GRAS domain with over 150 transcription factors in Arabidopsis thaliana. Besides, DELLAs from vascular plants can interact through the N-terminal domain with the gibberellin receptor encoded by GID1, through which gibberellins promote DELLA degradation. However, this regulation is absent in non-vascular land plants, which lack active gibberellins or a proper GID1 receptor. -

Download This Publication (PDF File)
PUBLIC LIBRARY of SCIENCE | plosgenetics.org | ISSN 1553-7390 | Volume 2 | Issue 12 | DECEMBER 2006 GENETICS PUBLIC LIBRARY of SCIENCE www.plosgenetics.org Volume 2 | Issue 12 | DECEMBER 2006 Interview Review Knight in Common Armor: 1949 Unraveling the Genetics 1956 An Interview with Sir John Sulston e225 of Human Obesity e188 Jane Gitschier David M. Mutch, Karine Clément Research Articles Natural Variants of AtHKT1 1964 The Complete Genome 2039 Enhance Na+ Accumulation e210 Sequence and Comparative e206 in Two Wild Populations of Genome Analysis of the High Arabidopsis Pathogenicity Yersinia Ana Rus, Ivan Baxter, enterocolitica Strain 8081 Balasubramaniam Muthukumar, Nicholas R. Thomson, Sarah Jeff Gustin, Brett Lahner, Elena Howard, Brendan W. Wren, Yakubova, David E. Salt Matthew T. G. Holden, Lisa Crossman, Gregory L. Challis, About the Cover Drosophila SPF45: A Bifunctional 1974 Carol Churcher, Karen The jigsaw image of representatives Protein with Roles in Both e178 Mungall, Karen Brooks, Tracey of various lines of eukaryote evolution Splicing and DNA Repair Chillingworth, Theresa Feltwell, refl ects the current lack of consensus as Ahmad Sami Chaouki, Helen K. Zahra Abdellah, Heidi Hauser, to how the major branches of eukaryotes Salz Kay Jagels, Mark Maddison, fi t together. The illustrations from upper Sharon Moule, Mandy Sanders, left to bottom right are as follows: a single Mammalian Small Nucleolar 1984 Sally Whitehead, Michael A. scale from the surface of Umbellosphaera; RNAs Are Mobile Genetic e205 Quail, Gordon Dougan, Julian Amoeba, the large amoeboid organism Elements Parkhill, Michael B. Prentice used as an introduction to protists for Michel J. Weber many school children; Euglena, the iconic Low Levels of Genetic 2052 fl agellate that is often used to challenge Soft Sweeps III: The Signature 1998 Divergence across e215 ideas of plants (Euglena has chloroplasts) of Positive Selection from e186 Geographically and and animals (Euglena moves); Stentor, Recurrent Mutation Linguistically Diverse one of the larger ciliates; Cacatua, the Pleuni S. -

Broadly Sampled Multigene Analyses Yield a Well-Resolved Eukaryotic Tree of Life
Smith ScholarWorks Biological Sciences: Faculty Publications Biological Sciences 10-1-2010 Broadly Sampled Multigene Analyses Yield a Well-Resolved Eukaryotic Tree of Life Laura Wegener Parfrey University of Massachusetts Amherst Jessica Grant Smith College Yonas I. Tekle Smith College Erica Lasek-Nesselquist Marine Biological Laboratory Hilary G. Morrison Marine Biological Laboratory See next page for additional authors Follow this and additional works at: https://scholarworks.smith.edu/bio_facpubs Part of the Biology Commons Recommended Citation Parfrey, Laura Wegener; Grant, Jessica; Tekle, Yonas I.; Lasek-Nesselquist, Erica; Morrison, Hilary G.; Sogin, Mitchell L.; Patterson, David J.; and Katz, Laura A., "Broadly Sampled Multigene Analyses Yield a Well-Resolved Eukaryotic Tree of Life" (2010). Biological Sciences: Faculty Publications, Smith College, Northampton, MA. https://scholarworks.smith.edu/bio_facpubs/126 This Article has been accepted for inclusion in Biological Sciences: Faculty Publications by an authorized administrator of Smith ScholarWorks. For more information, please contact [email protected] Authors Laura Wegener Parfrey, Jessica Grant, Yonas I. Tekle, Erica Lasek-Nesselquist, Hilary G. Morrison, Mitchell L. Sogin, David J. Patterson, and Laura A. Katz This article is available at Smith ScholarWorks: https://scholarworks.smith.edu/bio_facpubs/126 Syst. Biol. 59(5):518–533, 2010 c The Author(s) 2010. Published by Oxford University Press, on behalf of the Society of Systematic Biologists. All rights reserved. For Permissions, please email: [email protected] DOI:10.1093/sysbio/syq037 Advance Access publication on July 23, 2010 Broadly Sampled Multigene Analyses Yield a Well-Resolved Eukaryotic Tree of Life LAURA WEGENER PARFREY1,JESSICA GRANT2,YONAS I. TEKLE2,6,ERICA LASEK-NESSELQUIST3,4, 3 3 5 1,2, HILARY G. -

General Botany Lab Review Fungi, Algae, Bryophytes, Ferns & Fern Allies
General Botany Lab Review Fungi, Algae, Bryophytes, Ferns & Fern Allies You have looked at a lot of stuff – both live and via prepared slides. You’ve also labeled at least one Life Cycle Diagram for each of the groups. Know what your benchmarks are for a general life cycle diagram and be able to label them. I will not ask you to identify anything to species or genus; be able to identify things to “group” (i.e., ascomycete, bryophyta, etc.) Be able to identify growth form (e.g., unicell, filamentous, etc.). Recognize the differences between sexual and asexual reroductive structures. All questions will be multiple choice. Material looked at: UNIT 1: FUNGI EXERCISE 1: CHYTRIDS/ CHYTRIDOMYCOTA: Allmyces arbusculus – life and prepared slides EXERCISE 2: ZYGOMYCETES/ ZYGOMYCOTA: Rhizopus stolonifer – live and prepared slides EXERCISE 2: MYCORRHIZA and the GLOMEROMYCETES/ GLOMEROMYCOTA – prepared slides only EXERCISE 3: ASCOMYCETES/ ASCOMYCOTA Aspergillus sp., Penicillium sp., Saccharomyces cerevisiae, Peziza sp., Sordaria fimicola, and Morchella sp. – a mixture of live and prepared materials EXERCISE 4: BASIDIOMYCETES/BASIDIOMYCETES Agaricus, Coprinus, Cronartium (a rust), Ustilago (a smut) – slides, fresh, and dried EXERCISE 5: SLIME MOLDS – live and prepared Physarum EXERCISE 6: LICHENS – live and prepared slides be able to identify the various growth forms UNIT 2: ALGAE EXERCISE 1: CYANOBACTERIA Anabaena sp., Nostoc, and Oscillaroria – live and prepared material EXERCISE 2: SUPERGROUP EXCAVATA (Phylum Euglenophyta) – live and prepared material -

Algae & Marine Plants of Point Reyes
Algae & Marine Plants of Point Reyes Green Algae or Chlorophyta Genus/Species Common Name Acrosiphonia coalita Green rope, Tangled weed Blidingia minima Blidingia minima var. vexata Dwarf sea hair Bryopsis corticulans Cladophora columbiana Green tuft alga Codium fragile subsp. californicum Sea staghorn Codium setchellii Smooth spongy cushion, Green spongy cushion Trentepohlia aurea Ulva californica Ulva fenestrata Sea lettuce Ulva intestinalis Sea hair, Sea lettuce, Gutweed, Grass kelp Ulva linza Ulva taeniata Urospora sp. Brown Algae or Ochrophyta Genus/Species Common Name Alaria marginata Ribbon kelp, Winged kelp Analipus japonicus Fir branch seaweed, Sea fir Coilodesme californica Dactylosiphon bullosus Desmarestia herbacea Desmarestia latifrons Egregia menziesii Feather boa Fucus distichus Bladderwrack, Rockweed Haplogloia andersonii Anderson's gooey brown Laminaria setchellii Southern stiff-stiped kelp Laminaria sinclairii Leathesia marina Sea cauliflower Melanosiphon intestinalis Twisted sea tubes Nereocystis luetkeana Bull kelp, Bullwhip kelp, Bladder wrack, Edible kelp, Ribbon kelp Pelvetiopsis limitata Petalonia fascia False kelp Petrospongium rugosum Phaeostrophion irregulare Sand-scoured false kelp Pterygophora californica Woody-stemmed kelp, Stalked kelp, Walking kelp Ralfsia sp. Silvetia compressa Rockweed Stephanocystis osmundacea Page 1 of 4 Red Algae or Rhodophyta Genus/Species Common Name Ahnfeltia fastigiata Bushy Ahnfelt's seaweed Ahnfeltiopsis linearis Anisocladella pacifica Bangia sp. Bossiella dichotoma Bossiella -

Neoproterozoic Origin and Multiple Transitions to Macroscopic Growth in Green Seaweeds
Neoproterozoic origin and multiple transitions to macroscopic growth in green seaweeds Andrea Del Cortonaa,b,c,d,1, Christopher J. Jacksone, François Bucchinib,c, Michiel Van Belb,c, Sofie D’hondta, f g h i,j,k e Pavel Skaloud , Charles F. Delwiche , Andrew H. Knoll , John A. Raven , Heroen Verbruggen , Klaas Vandepoeleb,c,d,1,2, Olivier De Clercka,1,2, and Frederik Leliaerta,l,1,2 aDepartment of Biology, Phycology Research Group, Ghent University, 9000 Ghent, Belgium; bDepartment of Plant Biotechnology and Bioinformatics, Ghent University, 9052 Zwijnaarde, Belgium; cVlaams Instituut voor Biotechnologie Center for Plant Systems Biology, 9052 Zwijnaarde, Belgium; dBioinformatics Institute Ghent, Ghent University, 9052 Zwijnaarde, Belgium; eSchool of Biosciences, University of Melbourne, Melbourne, VIC 3010, Australia; fDepartment of Botany, Faculty of Science, Charles University, CZ-12800 Prague 2, Czech Republic; gDepartment of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD 20742; hDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138; iDivision of Plant Sciences, University of Dundee at the James Hutton Institute, Dundee DD2 5DA, United Kingdom; jSchool of Biological Sciences, University of Western Australia, WA 6009, Australia; kClimate Change Cluster, University of Technology, Ultimo, NSW 2006, Australia; and lMeise Botanic Garden, 1860 Meise, Belgium Edited by Pamela S. Soltis, University of Florida, Gainesville, FL, and approved December 13, 2019 (received for review June 11, 2019) The Neoproterozoic Era records the transition from a largely clear interpretation of how many times and when green seaweeds bacterial to a predominantly eukaryotic phototrophic world, creat- emerged from unicellular ancestors (8). ing the foundation for the complex benthic ecosystems that have There is general consensus that an early split in the evolution sustained Metazoa from the Ediacaran Period onward. -

1 Integrative Biology 200 "PRINCIPLES OF
Integrative Biology 200 "PRINCIPLES OF PHYLOGENETICS" Spring 2018 University of California, Berkeley B.D. Mishler March 14, 2018. Classification II: Phylogenetic taxonomy including incorporation of fossils; PhyloCode I. Phylogenetic Taxonomy - the argument for rank-free classification A number of recent calls have been made for the reformation of the Linnaean hierarchy (e.g., De Queiroz & Gauthier, 1992). These authors have emphasized that the existing system is based in a non-evolutionary world-view; the roots of the Linnaean hierarchy are in a specially- created world-view. Perhaps the idea of fixed, comparable ranks made some sense under that view, but under an evolutionary world view they don't make sense. There are several problems with the current nomenclatorial system: 1. The current system, with its single type for a name, cannot be used to precisely name a clade. E.g., you may name a family based on a certain type specimen, and even if you were clear about what node you meant to name in your original publication, the exact phylogenetic application of your name would not be clear subsequently, after new clades are added. 2. There are not nearly enough ranks to name the thousands of levels of monophyletic groups in the tree of life. Therefore people are increasingly using informal rank-free names for higher- level nodes, but without any clear, formal specification of what clade is meant. 3. Most aspects of the current code, including priority, revolve around the ranks, which leads to instability of usage. For example, when a change in relationships is discovered, several names often need to be changed to adjust, including those of groups whose circumscription has not changed. -

Is Chloroplastic Class IIA Aldolase a Marine Enzyme&Quest;
The ISME Journal (2016) 10, 2767–2772 © 2016 International Society for Microbial Ecology All rights reserved 1751-7362/16 www.nature.com/ismej SHORT COMMUNICATION Is chloroplastic class IIA aldolase a marine enzyme? Hitoshi Miyasaka1, Takeru Ogata1, Satoshi Tanaka2, Takeshi Ohama3, Sanae Kano4, Fujiwara Kazuhiro4,7, Shuhei Hayashi1, Shinjiro Yamamoto1, Hiro Takahashi5, Hideyuki Matsuura6 and Kazumasa Hirata6 1Department of Applied Life Science, Sojo University, Kumamoto, Japan; 2The Kansai Electric Power Co., Environmental Research Center, Keihanna-Plaza, Kyoto, Japan; 3School of Environmental Science and Engineering, Kochi University of Technology, Kochi, Japan; 4Chugai Technos Corporation, Hiroshima, Japan; 5Graduate School of Horticulture, Faculty of Horticulture, Chiba University, Chiba, Japan and 6Environmental Biotechnology Laboratory, Graduate School of Pharmaceutical Sciences, Osaka University, Osaka, Japan Expressed sequence tag analyses revealed that two marine Chlorophyceae green algae, Chlamydo- monas sp. W80 and Chlamydomonas sp. HS5, contain genes coding for chloroplastic class IIA aldolase (fructose-1, 6-bisphosphate aldolase: FBA). These genes show robust monophyly with those of the marine Prasinophyceae algae genera Micromonas, Ostreococcus and Bathycoccus, indicating that the acquisition of this gene through horizontal gene transfer by an ancestor of the green algal lineage occurred prior to the divergence of the core chlorophytes (Chlorophyceae and Treboux- iophyceae) and the prasinophytes. The absence of this gene in some freshwater chlorophytes, such as Chlamydomonas reinhardtii, Volvox carteri, Chlorella vulgaris, Chlorella variabilis and Coccomyxa subellipsoidea, can therefore be explained by the loss of this gene somewhere in the evolutionary process. Our survey on the distribution of this gene in genomic and transcriptome databases suggests that this gene occurs almost exclusively in marine algae, with a few exceptions, and as such, we propose that chloroplastic class IIA FBA is a marine environment-adapted enzyme. -

CH28 PROTISTS.Pptx
9/29/14 Biosc 41 Announcements 9/29 Review: History of Life v Quick review followed by lecture quiz (history & v How long ago is Earth thought to have formed? phylogeny) v What is thought to have been the first genetic material? v Lecture: Protists v Are we tetrapods? v Lab: Protozoa (animal-like protists) v Most atmospheric oxygen comes from photosynthesis v Lab exam 1 is Wed! (does not cover today’s lab) § Since many of the first organisms were photosynthetic (i.e. cyanobacteria), a LOT of excess oxygen accumulated (O2 revolution) § Some organisms adapted to use it (aerobic respiration) Review: History of Life Review: Phylogeny v Which organelles are thought to have originated as v Homology is similarity due to shared ancestry endosymbionts? v Analogy is similarity due to convergent evolution v During what event did fossils resembling modern taxa suddenly appear en masse? v A valid clade is monophyletic, meaning it consists of the ancestor taxon and all its descendants v How many mass extinctions seem to have occurred during v A paraphyletic grouping consists of an ancestral species and Earth’s history? Describe one? some, but not all, of the descendants v When is adaptive radiation likely to occur? v A polyphyletic grouping includes distantly related species but does not include their most recent common ancestor v Maximum parsimony assumes the tree requiring the fewest evolutionary events is most likely Quiz 3 (History and Phylogeny) BIOSC 041 1. How long ago is Earth thought to have formed? 2. Why might many organisms have evolved to use aerobic respiration? PROTISTS! Reference: Chapter 28 3. -
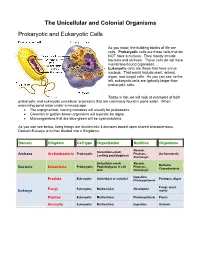
The Unicellular and Colonial Organisms Prokaryotic And
The Unicellular and Colonial Organisms Prokaryotic and Eukaryotic Cells As you know, the building blocks of life are cells. Prokaryotic cells are those cells that do NOT have a nucleus. They mostly include bacteria and archaea. These cells do not have membrane-bound organelles. Eukaryotic cells are those that have a true nucleus. That would include plant, animal, algae, and fungal cells. As you can see, to the left, eukaryotic cells are typically larger than prokaryotic cells. Today in lab, we will look at examples of both prokaryotic and eukaryotic unicellular organisms that are commonly found in pond water. When examining pond water under a microscope… The unpigmented, moving microbes will usually be protozoans. Greenish or golden-brown organisms will typically be algae. Microorganisms that are blue-green will be cyanobacteria. As you can see below, living things are divided into 3 domains based upon shared characteristics. Domain Eukarya is further divided into 4 Kingdoms. Domain Kingdom Cell type Organization Nutrition Organisms Absorb, Unicellular-small; Prokaryotic Photsyn., Archaeacteria Archaea Archaebacteria Lacking peptidoglycan Chemosyn. Unicellular-small; Absorb, Bacteria, Prokaryotic Peptidoglycan in cell Photsyn., Bacteria Eubacteria Cyanobacteria wall Chemosyn. Ingestion, Eukaryotic Unicellular or colonial Protozoa, Algae Protista Photosynthesis Fungi, yeast, Fungi Eukaryotic Multicellular Absorption Eukarya molds Plantae Eukaryotic Multicellular Photosynthesis Plants Animalia Eukaryotic Multicellular Ingestion Animals Prokaryotic Organisms – the archaea, non-photosynthetic bacteria, and cyanobacteria Archaea - Microorganisms that resemble bacteria, but are different from them in certain aspects. Archaea cell walls do not include the macromolecule peptidoglycan, which is always found in the cell walls of bacteria. Archaea usually live in extreme, often very hot or salty environments, such as hot mineral springs or deep-sea hydrothermal vents. -

The Origin of Alternation of Generations in Land Plants
Theoriginof alternation of generations inlandplants: afocuson matrotrophy andhexose transport Linda K.E.Graham and LeeW .Wilcox Department of Botany,University of Wisconsin, 430Lincoln Drive, Madison,WI 53706, USA (lkgraham@facsta¡.wisc .edu ) Alifehistory involving alternation of two developmentally associated, multicellular generations (sporophyteand gametophyte) is anautapomorphy of embryophytes (bryophytes + vascularplants) . Microfossil dataindicate that Mid ^Late Ordovicianland plants possessed such alifecycle, and that the originof alternationof generationspreceded this date.Molecular phylogenetic data unambiguously relate charophyceangreen algae to the ancestryof monophyletic embryophytes, and identify bryophytes as early-divergentland plants. Comparison of reproduction in charophyceans and bryophytes suggests that the followingstages occurredduring evolutionary origin of embryophytic alternation of generations: (i) originof oogamy;(ii) retention ofeggsand zygotes on the parentalthallus; (iii) originof matrotrophy (regulatedtransfer ofnutritional and morphogenetic solutes fromparental cells tothe nextgeneration); (iv)origin of a multicellularsporophyte generation ;and(v) origin of non-£ agellate, walled spores. Oogamy,egg/zygoteretention andmatrotrophy characterize at least some moderncharophyceans, and arepostulated to represent pre-adaptativefeatures inherited byembryophytes from ancestral charophyceans.Matrotrophy is hypothesizedto have preceded originof the multicellularsporophytes of plants,and to represent acritical innovation.Molecular -

JUDD W.S. Et. Al. (2002) Plant Systematics: a Phylogenetic Approach. Chapter 7. an Overview of Green
UNCORRECTED PAGE PROOFS An Overview of Green Plant Phylogeny he word plant is commonly used to refer to any auto- trophic eukaryotic organism capable of converting light energy into chemical energy via the process of photosynthe- sis. More specifically, these organisms produce carbohydrates from carbon dioxide and water in the presence of chlorophyll inside of organelles called chloroplasts. Sometimes the term plant is extended to include autotrophic prokaryotic forms, especially the (eu)bacterial lineage known as the cyanobacteria (or blue- green algae). Many traditional botany textbooks even include the fungi, which differ dramatically in being heterotrophic eukaryotic organisms that enzymatically break down living or dead organic material and then absorb the simpler products. Fungi appear to be more closely related to animals, another lineage of heterotrophs characterized by eating other organisms and digesting them inter- nally. In this chapter we first briefly discuss the origin and evolution of several separately evolved plant lineages, both to acquaint you with these important branches of the tree of life and to help put the green plant lineage in broad phylogenetic perspective. We then focus attention on the evolution of green plants, emphasizing sev- eral critical transitions. Specifically, we concentrate on the origins of land plants (embryophytes), of vascular plants (tracheophytes), of 1 UNCORRECTED PAGE PROOFS 2 CHAPTER SEVEN seed plants (spermatophytes), and of flowering plants dons.” In some cases it is possible to abandon such (angiosperms). names entirely, but in others it is tempting to retain Although knowledge of fossil plants is critical to a them, either as common names for certain forms of orga- deep understanding of each of these shifts and some key nization (e.g., the “bryophytic” life cycle), or to refer to a fossils are mentioned, much of our discussion focuses on clade (e.g., applying “gymnosperms” to a hypothesized extant groups.