An Audit of Performance in the Processing of Macro-Invertebrate Samples in 1991
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environmental Protection Final Draft Report
Environmental Protection Final Draft Report ANNUAL CLASSIFICATION OF RIVER WATER QUALITY 1992: NUMBERS OF SAMPLES EXCEEDING THE QUALITY STANDARD June 1993 FWS/93/012 Author: R J Broome Freshwater Scientist NRA C.V.M. Davies National Rivers Authority Environmental Protection Manager South West R egion ANNUAL CLASSIFICATION OF RIVER WATER QUALITY 1992: NUMBERS OF SAMPLES EXCEEDING TOE QUALITY STANDARD - FWS/93/012 This report shows the number of samples taken and the frequency with which individual determinand values failed to comply with National Water Council river classification standards, at routinely monitored river sites during the 1992 classification period. Compliance was assessed at all sites against the quality criterion for each determinand relevant to the River Water Quality Objective (RQO) of that site. The criterion are shown in Table 1. A dashed line in the schedule indicates no samples failed to comply. This report should be read in conjunction with Water Quality Technical note FWS/93/005, entitled: River Water Quality 1991, Classification by Determinand? where for each site the classification for each individual determinand is given, together with relevant statistics. The results are grouped in catchments for easy reference, commencing with the most south easterly catchments in the region and progressing sequentially around the coast to the most north easterly catchment. ENVIRONMENT AGENCY 110221i i i H i m NATIONAL RIVERS AUTHORITY - 80UTH WEST REGION 1992 RIVER WATER QUALITY CLASSIFICATION NUMBER OF SAMPLES (N) AND NUMBER -

Environmentol Protection Report WATER QUALITY MONITORING
5k Environmentol Protection Report WATER QUALITY MONITORING LOCATIONS 1992 April 1992 FW P/9 2/ 0 0 1 Author: B Steele Technicol Assistant, Freshwater NRA National Rivers Authority CVM Davies South West Region Environmental Protection Manager HATER QUALITY MONITORING LOCATIONS 1992 _ . - - TECHNICAL REPORT NO: FWP/92/001 The maps in this report indicate the monitoring locations for the 1992 Regional Water Quality Monitoring Programme which is described separately. The presentation of all monitoring features into these catchment maps will assist in developing an integrated approach to catchment management and operation. The water quality monitoring maps and index were originally incorporated into the Catchment Action Plans. They provide a visual presentation of monitored sites within a catchment and enable water quality data to be accessed easily by all departments and external organisations. The maps bring together information from different sections within Water Quality. The routine river monitoring and tidal water monitoring points, the licensed waste disposal sites and the monitored effluent discharges (pic, non-plc, fish farms, COPA Variation Order [non-plc and pic]) are plotted. The type of discharge is identified such as sewage effluent, dairy factory, etc. Additionally, river impact and control sites are indicated for significant effluent discharges. If the watercourse is not sampled then the location symbol is qualified by (*). Additional details give the type of monitoring undertaken at sites (ie chemical, biological and algological) and whether they are analysed for more specialised substances as required by: a. EC Dangerous Substances Directive b. EC Freshwater Fish Water Quality Directive c. DOE Harmonised Monitoring Scheme d. DOE Red List Reduction Programme c. -

River Water Quality 1992 Classification by Determinand
N f\A - S oo-Ha (jO$*\z'3'Z2 Environmental Protection Final Draft Report RIVER WATER QUALITY 1992 CLASSIFICATION BY DETERMINAND May 1993 Water Quality Technical Note FWS/93/005 Author: R J Broome Freshwater Scientist NRA CV.M. Davies National Rivers A h ority Environmental Protection Manager South West Region RIVER WATER QUALITY 1992 CLASSIFICATION BY DETERMINAND 1. INTRODUCTION River water quality is monitored in 34 catchments in the region. Samples are collected at a minimum frequency of once a month from 422 watercourses at 890 locations within the Regional Monitoring Network. Each sample is analysed for a range of chemical and physical determinands. These sample results are stored in the Water Quality Archive. A computerised system assigns a quality class to each monitoring location and associated upstream river reach. This report contains the results of the 1992 river water quality classifications for each determinand used in the classification process. 2. RIVER WATER QUALITY ASSESSMENT The assessment of river water quality is by comparison of current water quality against River Quality Objectives (RQO's) which have been set for many river lengths in the region. Individual determinands have been classified in accordance with the requirements of the National Water Council (NWC) river classification system which identifies river water quality as being one of five classes as shown in Table 1 below: TABLE 1 NATIONAL WATER COUNCIL - CLASSIFICATION SYSTEM CLASS DESCRIPTION 1A Good quality IB Lesser good quality 2 Fair quality 3 Poor quality 4 Bad quality The classification criteria used for attributing a quality class to each criteria are shown in Appendix 1. -

The Bryophytes of Cornwall and the Isles of Scilly
THE BRYOPHYTES OF CORNWALL AND THE ISLES OF SCILLY by David T. Holyoak Contents Acknowledgements ................................................................................ 2 INTRODUCTION ................................................................................. 3 Scope and aims .......................................................................... 3 Coverage and treatment of old records ...................................... 3 Recording since 1993 ................................................................ 5 Presentation of data ................................................................... 6 NOTES ON SPECIES .......................................................................... 8 Introduction and abbreviations ................................................. 8 Hornworts (Anthocerotophyta) ................................................. 15 Liverworts (Marchantiophyta) ................................................. 17 Mosses (Bryophyta) ................................................................. 98 COASTAL INFLUENCES ON BRYOPHYTE DISTRIBUTION ..... 348 ANALYSIS OF CHANGES IN BRYOPHYTE DISTRIBUTION ..... 367 BIBLIOGRAPHY ................................................................................ 394 1 Acknowledgements Mrs Jean A. Paton MBE is thanked for use of records, gifts and checking of specimens, teaching me to identify liverworts, and expertise freely shared. Records have been used from the Biological Records Centre (Wallingford): thanks are due to Dr M.O. Hill and Dr C.D. Preston for -

Quethiock Neighbourhood Development Plan
General Conformity Statement: Quethiock Neighbourhood Development Plan Following the Adoption of the Cornwall Local Plan: Strategic Policies on 22nd November 2016, any Neighbourhood Development Plan ‘made’ prior to that date has been checked against the policies of the Cornwall Local Plan for general conformity. The Quethiock NDP has been assessed to determine whether any of its policies would be in conflict with the policies of the Cornwall Local Plan All policies within the Quethiock NDP are considered to be in general conformity with the Cornwall Local Plan: Strategic Policies and should be used in determining planning applications in the NDP area. 1 QUETHIOCK PARISH NEIGHBOURHOOD DEVELOPMENT PLAN 2010 – 2030 Table of Contents Page No FOREWORD 2 1. INTRODUCTION 3 2. A DESCRIPTION OF QUETHIOCK PARISH 4 3. THE VIEW OF THE COMMUNITY 6 4. THE NPPF, CARADON AND CORNWALL LOCAL PLANS 7 5. VISION AND OBJECTIVES 7 6. POLICIES 8 HOUSING POLICY 9 Policy H1: Housing Development 9 BUSINESS POLICIES 11 Policy B1: Small Business – Change of Use 11 Policy B2: Small Business – New Build 12 COMMUNITY POLICIES 13 Policy C1: Quethiock Pavilion 13 Policy C2: Children’s Play Area 14 Policy C3: Quethiock School Playing Field 14 6th May 2015 2 QUETHIOCK VILLAGE FOREWORD The process of creating the Quethiock Neighbourhood Development Plan (NDP) has been led by members of the community and is part of the Government’s recently revised approach to planning contained in the Localism Act of 2011 i.e. local people have more say about what happens in the area in which they live. The aim of this NDP is to put forward the wishes of the community as to any future development. -
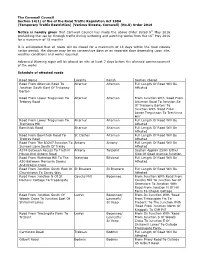
Surfacing & Patching Works
The Cornwall Council Section 14(1) of the of the Road Traffic Regulation Act 1984 (Temporary Traffic Restriction) (Various Streets, Cornwall) (No.8) Order 2016 Notice is hereby given that Cornwall Council has made the above Order dated 9th May 2016 prohibiting the use by through traffic during surfacing and patching works from the 16 th May 2016 for a maximum of 18 months It is anticipated that all roads will be closed for a maximum of 14 days within the road closure notice period; the closure may be on consecutive days or on separate days depending upon site, weather conditions and works required. Advanced Warning signs will be placed on site at least 7 days before the planned commencement of the works Schedule of affected roads Road Name Locality Parish Section Closed Road From Altarnun Road To Altarnun Altarnun Full Length Of Road Will Be Junction South East Of Trelawny Affected Barton Road From Lower Tregunnon To Altarnun Altarnun From Junction With 'Road From Trebray Road Altarnun Road To Junction Se Of Trelawny Barton' To Junction With 'Road From Lower Tregunnon To Trethinna Hill' Road From Lower Tregunnon To Altarnun Altarnun Full Length Of Road Will Be Trethinna Hill Affected Bowithick Road Altarnun Altarnun Full Length Of Road Will Be Affected Road From Bowithick Road To St Clether Altarnun Full Length Of Road Will Be Trebray Road Affected Road From The B3247 Junction To Antony Antony Full Length Of Road Will Be Sunwell Lane South Of Trelay Affected A374 Between Access To Trethill Antony Torpoint Section Approx 250m Either House -

Penwith Statement 2 February 1998
CORNWALL COUNTY COUNCIL PUBLIC RIGHTS OF WAY NATIONAL PARKS AND ACCESS TO THE COUNTRYSIDE ACT 1949 COUNTRYSIDE ACT 1968 WILDLIFE AND COUNTRYSIDE ACT 1981 REVISED STATEMENT PENWITH DISTRICT Parish of GWINEAR-GWITHIAN Relevant date for the purposes of this revised Definitive Statement: 2nd February 1998 _______________________________________________________________________________________________________________________ NO. LOCATION AVERAGE MIN WIDTH WIDTH _______________________________________________________________________________________________________________________ 1 FP from road west of Barripper to Coswinsawsin Lane 3'0" 2 FP from road south west of Carnhell Green to BR 49 at Cathebedron 3'0" 3 FP from Shaft Downs to BR 49 3'0" 4 FP from road south of Halancoose to B3280 3'0" 5 FP from BR 49 south of Drewollas to FP 6 3'0" 6 FP from BR 49 north east of Gwinear Downs to FP 5 2'6" 7 FP from road south of Deveral to BW 52 west of Calloose - 8 FP from south of Taskus to Parish Boundary 2'6" 9 FP from BR 54 at Trenerth to BW 52 at Calloose Caravan Park 2'0" 1.0m 10 FP from Tregotha to Parish Boundary and Hayle FP 44 - 11 FP from south of Gwinear to Deverell Road west of Henvor 2'6" 12 FP from BR 49 at Drewollas to Reawla Lane (Wall) 2'6" 13 FP from Gwinear to road north of Relistien 3'0" 14 FP from Rosewarne to Lanyon Gate 3'0" 15 FP from Lanyon Gate to road north of Carnhell Green - 16 FP and BR from Gwinear via Lanyon Farm to former Gwinear Road Station 3'0" 1.5m 17 FP from Higher Trevaskis (BR16) to lane west of Trevaskis 2'6" 18 FP from BR 16 north of Lanyon to south of Trenowin 2'6" 19 FP from Gwinear to Polkinghorne 2'6" 20 FP from Gwinear via Trungle to Parish Boundary at Angarrack 3'0" Parish of GWINEAR-GWITHIAN Relevant Date 2nd February 1998 - Sheet 2 _______________________________________________________________________________________________________________________ NO. -

CORNWALL. FIR 1141 ''Rilliams William, Penpoll, St
TRADES DIRECTORY.] CORNWALL. FIR 1141 ''rilliams William, Penpoll, St. Feock, Woodley J. Penstraze, Kenwyn, Truro fDunbarWilliam (to Capt. C. Dudley Fol"- Devoran R.S.O Woodley John, Wood tenement, St tesctte), Ford, Boconnoc, Lostwithiel Williams William, Penponds, Camborne Enoder, Grampound Road R.S.O Grigg John (to jJf. H. Williams e.~q ), Williams William, Rosemelling, Luxul- W oodley Samuel, Hill park, Kilkhamp- Arrallas, St. Enoder, Grampound Rd yan, Lostwithiel ton, Stratton Harris Christopher (to ~Jfr. Hitchins) Williams William, St. Blazey, Par Woodley Thos. Carland, St. Erme,Trnro Pressingole, St. Agnes, Scorrier R.S.6 station R.S.O Woodward Robert, Lelant R.S.O Hicks Richard (to .JJfessr:J. Dingley, Williams William, St. Tudy, Bodmin Woodward S. Trenince, St. Issey R.S.O Peth.ybridge, White '.f Din.qley), Vacye Williams William, Stonaford, North Woodward T. J.Tregonce,St. Issey R.S.O house, North Tamerton, Holsworthy hill, Launceston Wood wards Charles, Trequite, St. Ke,v, Irons John (to William Teague esq.), Ad- Williams William, Trewellard, Pendeen, Wadebridge R.S.O vent, Camelford Penzance Woolcock William & Hugh, Sellan, Jacka James (to E. Bolitho esq.), Trer Williams William Edward, Prislow, Sancreed, Penzance nuggo, Sancreed, Penza.nce Budock, Falmouth Woolcock Henry, Tresidder, St. Buryan, Julian John (to ~lfr. Walter Hick:J, of Willoughby Mrs. Elizabeth, Blackwater, Penzance St. Austell), Lower Menadue, Luxul- Mithian, Scorrier RS.O Woolcock H. Trispin, St. Erme, Truro yan, Lnstwithiel Willoughby Henry, Menegissey, Mount Woolcock Jn. Golden, Grampound Road Keat John (to William Teague esq.), Hawke, Scorrier R.S.O "\Voolcock J.Noonvares,Leeds twn.Hayle Kcloader,St.Minver,WadebriJge R.S.O Willoughby John, Chf'garder, Redruth Woolcock Philip, Kestle mill, Newlyn, Ley John (to Thomas Greemoood esq.), Willoughby Thomas, Tehidy Barton, Grampound Road St. -
![CORNWALL.] FARMERS Continued](https://docslib.b-cdn.net/cover/9312/cornwall-farmers-continued-2239312.webp)
CORNWALL.] FARMERS Continued
TRADES DIRECTORY.] 959 FAR [CORNWALL.] FARMERS continued. Treffry Mrs. Grace, Peneskin, Ruan TrembathJames, Carnequidden & Bos- Tbomas Thomas, Mudianvean, St. Laniborne, Grampouod crege, Gulval, Penzance Martin-in-Meneage, Helston Treffry J.GovilyMinor,Cuby,Grrnpound Trembath James, Menwidden, Ludgvan, Thomas Tbos. Penmarth, Carnmenellis, Tregaskis Samuel, Tregonce, St. Issey Penzance Redruth Tregear Edward,Green,St.Mary's,Scilly Trembath J. Ninnis, Gwennap, Redruth Thomas Thos.Spittal,St.Mabyn,Bodmin Tregear James, Boswarlas, St. Just-in- Trembath J.Tremhath, Morvah,Penznce ThomasW.Bojewyan,Pendeen,Penzance Penwith, Penzance Trembath John, Kenwyn, Truro Thomas Wm. Cadwin, Lanivet, Bodmiu TregearJohn,Pentreath,Breage,Helston Trembath John, Pendeen, Penzance Thomas William, Godolphin, Helston Tregear William, Boswarlas, St. Just- Trembath Matthew,Bojewyan,Pendeen, Thomas W.Lidcott, Cardinham, Bodmin in-Penwith, Penzance Penzance Thomas W. Mulberry, Lanivet, Bodmin Tregear.William, Green,St.Mary's,Scilly Trembath Richard, Pedneventon, Thomas William, N anpean, St. Tregellas J .Church tn. St.Agnes,Scorrier Madron, Penzance Stephens-in-Branwell Tregenza T. Mylor brdg. Mylor, Falmth Trembath Richard, Trevilley,St.Sennen, Thomas W . .Ninnis, Wendron, Helston Treglown Henry, Bolenowe, Treslothan, Penzance Thomas William, Park Ventonsah, Camborne Trembath William, Tresvennack, St. Mullion, Helst.on TregoningArchelaus,Hugos, Kea, Truro Paul, Penzance Thomas W. Trereen, Zennor, St. Ives Tregoning Mrs. Elizabeth, Colvadnack, Tremhath William David, Portb, St. Thomas W.Wheel Wreeth,Lelant,Hayle Carnmenellis, Redruth Anthony-in-Roseland, Gramponnd Thomas Wm. Zelah, St. Alien, Truro Tregoning Henry, Tregilsoe, St. Hilary, Tremewan William,Horrows,Luxulyan, Thomas W. H. Higher Market st. Penryn M arazion Bodmin Thomas William Henry, Lancarrow, Tregoning Mrs. Maria, Walkey trees, TremewenNicholas, Trewey, St. Levan, Carnmenellis, Redruth St. -

Display PDF in Separate
environment agency plan TAMAR ANNUAL REVIEW 2000 December 2000 HOLSWORTHY LAUNCESTOI LISKEARD LOOE FOWEY PLYMOUTH FALMOUTH En v ir o n m e n t Ag e n c y Tamar Annual Review 2000 Further copies of this Annual Review can be obtained from: Team Leader, LEAPs Environment Agency Sir John Moore House Victoria Square Bodmin PL31 1EB Environment Agency Copyright Waiver This report is Intended to be used widely and may be quoted, copied or reproduced In any way; provided that the extracts are not quoted out of context and that due acknowledgement Is given to the Environment Agency. Note: This Is not a legally or scientifically binding document. ENVIRONMENT A G EN C Y 109199 Tamar Annual Review 2000 Our Vision Our vision is of this area being managed in a sustainable way, that balances the needs of all users with the needs of the environment. We look forward to a future where a healthy economy leads to: Biodiversity and the physical habitat for wildlife being enhanced People’s enjoyment and appreciation of the environment continuing to grow Pressures from human needs being satisfied sustainably Foreword This is the combined Annual Review of the Tamar Estuary and Freshwater Tamar Action Plans. It describes the progress that has been made since. In addition to our own actions in the plan area we welcome opportunities to work in partnership with other groups. Tamar Annual Review 2000 Contents 1. Introduction 2. Area Overview 3. Protection through Partnership 4. Actions 4.1 Quality of surface waters and groundwaters 4.1.1 Effects of effluent discharges -

Sancreed Parish Plan
SANCREED PARISH PLAN MARCH 2009 Contents 1. Vision Statement and Introduction 2. Purpose of the Parish Plan 3. The People 4. Traffic and Transport 5. The Environment 6. Community Life 7. Major Concerns 8. Identified Concerns for Action 9. Action Plans 10. What Happens Next Vision Statement A parish with an enhanced sense of awareness, identity and feeling of community. The Parish of Sancreed Sancreed Parish is a beautiful inland rural parish situated in the heart of West Penwith, some three to four miles west of Penzance. The only parish in Penwith that does not border the sea, but of an open aspect with far reaching views over the surrounding countryside, it overlooks both Mounts Bay and the Atlantic Ocean beyond Pendeen. With much of its land falling within an Area of Outstanding Natural Beauty and within an Environmentally Sensitive Area, Sancreed boasts much natural beauty which delights residents and visitors alike. Sancreed possesses three main villages. To one side of the Parish is Newbridge, situated along the A3071 to St Just, and to the other, on the A30 to Lands End, is the smaller village of Drift. Sancreed (Churchtown), with its historic church, is situated in between. The remainder of Sancreed Parish consists of smaller hamlets: Brane, Catchall, Grumbla, Sellan, Tregerest, Tregonebris; outlying farms and open moorland. Being of a rural and widespread nature, Sancreed Parish possesses no defined centre and lacks amenities. Newbridge possesses the only public house, the Fountain Inn. There are garages both at Newbridge and Drift. Methodist Chapels can be found at Drift and Tregerest. -

MAP 2 Proposed Electoral Divisions in Penzance and Madron
SHEET 2, MAP 2 Proposed Electoral Divisions in Penzance and Madron ST IVES WEST ED TOWEDNACK CP ZENNOR CP MORVAH CP Carnaquidden Downs Water THE BOUNDARY COMMITTEE FOR ENGLAND ELECTORAL REVIEW OF CORNWALL Higher Trenowin Trenowin Downs Spoil Heap Final Recommendations for Electoral Division Boundaries in the Unitary Authority of Cornwall December 2009 Sheet 2 of 20 Great Downs This map is based upon Ordnance Survey material with the permission of Ordnance Survey on behalf of the Controller of Her Majesty's Stationery Office © Crown copyright. Unauthorised reproduction infringes Crown copyright and may lead to prosecution or civil proceedings. Chysauster The Electoral Commission GD03114G 2009. Settlement Tredinneck Mulfra Boskednan Castle an Dinas Quarry 11 33 (Granite) B Ding Dong Mine (disused) Higher Ninnes Settlement Bay of Biscay Lower Ninnes Trythall Junior & Lanyon Farm Carfury Newmill Infant School GULVAL R o s Trezelah Park PARISH WARD e m Farm o r r Boscreege a n C A Farm S S t r T e L Bosilliack a E m R O A D Crankan LUDGVAN ED B 3 309 G EA Lower Garris R LA NE Higher Tremenheere T Farm re v a y LUDGVAN CP lo r S t re a m Tremhea Badger's Cross Barn Boswarthen T R E MADRON CP G A S S A C K R O A D C h y a Higher Boscobba Farm n d o u r B ro o k Kennels Cricket Kenegie Manor Ground (Hotel & Holiday Village) Tolver Farm L L I H Y R Playing R Field A Higher Trewern U Q 1 1 3 3 Little B E MADRON N Lower Trewern Rosemorran A L Farm N PARISH WARD R FO E RE W E ST R REE T T Cemy Madron Boskenwyn Boscrowan Manor SCHOO Madron L LANE (Daniel's