Seasonal Variations of Water Characteristics in Three Urban Ponds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
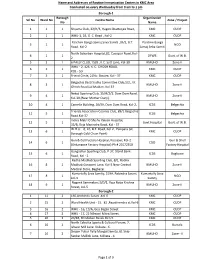
Name and Addresses of Routine Immunization Centers in KMC Area
Name and Addresses of Routine Immunization Centers in KMC Area Conducted on every Wednesday from 9 am to 1 pm Borough-1 Borough Organization Srl No Ward No Centre Name Zone / Project No Name 1 1 1 Shyama Club, 22/H/3, Hagen Chatterjee Road, KMC CUDP 2 1 1 WHU-1, 1B, G. C. Road , Kol-2 KMC CUDP Paschim Banga Samaj Seva Samiti ,35/2, B.T. Paschim Banga 3 1 1 NGO Road, Kol-2 Samaj Seba Samiti North Subarban Hospital,82, Cossipur Road, Kol- 4 1 1 DFWB Govt. of W.B. 2 5 2 1 6 PALLY CLUB, 15/B , K.C. Sett Lane, Kol-30 KMUHO Zone-II WHU - 2, 126, K. C. GHOSH ROAD, 6 2 1 KMC CUDP KOL - 50 7 3 1 Friend Circle, 21No. Bustee, Kol - 37 KMC CUDP Belgachia Basti Sudha Committee Club,1/2, J.K. 8 3 1 KMUHO Zone-II Ghosh Road,Lal Maidan, Kol-37 Netaji Sporting Club, 15/H/2/1, Dum Dum Road, 9 4 1 KMUHO Zone-II Kol-30,(Near Mother Diary). 10 4 1 Camelia Building, 26/59, Dum Dum Road, Kol-2, ICDS Belgachia Friends Association Cosmos Club, 89/1 Belgachia 11 5 1 ICDS Belgachia Road.Kol-37 Indira Matri O Shishu Kalyan Hospital, 12 5 1 Govt.Hospital Govt. of W.B. 35/B, Raja Manindra Road, Kol - 37 W.H.U. - 6, 10, B.T. Road, Kol-2 , Paikpara (at 13 6 1 KMC CUDP Borough Cold Chain Point) Gun & Cell Factory Hospital, Kossipur, Kol-2 Gun & Shell 14 6 1 CGO (Ordanance Factory Hospital) Ph # 25572350 Factory Hospital Gangadhar Sporting Club, P-37, Stand Bank 15 6 1 ICDS Bagbazar Road, Kol - 2 Radha Madhab Sporting Club, 8/1, Radha 16 8 1 Madhab Goswami Lane, Kol-3.Near Central KMUHO Zone-II Medical Store, Bagbazar Kumartully Seva Samity, 519A, Rabindra Sarani, Kumartully Seva 17 8 1 NGO kol-3 Samity Nagarik Sammelani,3/D/1, Raja Naba Krishna 18 9 1 KMUHO Zone-II Street, kol-5 Borough-2 1 11 2 160,Arobindu Sarani ,Kol-6 KMC CUDP 2 15 2 Ward Health Unit - 15. -

W.B.C.S.(Exe.) Officers of West Bengal Cadre
W.B.C.S.(EXE.) OFFICERS OF WEST BENGAL CADRE Sl Name/Idcode Batch Present Posting Posting Address Mobile/Email No. 1 ARUN KUMAR 1985 COMPULSORY WAITING NABANNA ,SARAT CHATTERJEE 9432877230 SINGH PERSONNEL AND ROAD ,SHIBPUR, (CS1985028 ) ADMINISTRATIVE REFORMS & HOWRAH-711102 Dob- 14-01-1962 E-GOVERNANCE DEPTT. 2 SUVENDU GHOSH 1990 ADDITIONAL DIRECTOR B 18/204, A-B CONNECTOR, +918902267252 (CS1990027 ) B.R.A.I.P.R.D. (TRAINING) KALYANI ,NADIA, WEST suvendughoshsiprd Dob- 21-06-1960 BENGAL 741251 ,PHONE:033 2582 @gmail.com 8161 3 NAMITA ROY 1990 JT. SECY & EX. OFFICIO NABANNA ,14TH FLOOR, 325, +919433746563 MALLICK DIRECTOR SARAT CHATTERJEE (CS1990036 ) INFORMATION & CULTURAL ROAD,HOWRAH-711102 Dob- 28-09-1961 AFFAIRS DEPTT. ,PHONE:2214- 5555,2214-3101 4 MD. ABDUL GANI 1991 SPECIAL SECRETARY MAYUKH BHAVAN, 4TH FLOOR, +919836041082 (CS1991051 ) SUNDARBAN AFFAIRS DEPTT. BIDHANNAGAR, mdabdulgani61@gm Dob- 08-02-1961 KOLKATA-700091 ,PHONE: ail.com 033-2337-3544 5 PARTHA SARATHI 1991 ASSISTANT COMMISSIONER COURT BUILDING, MATHER 9434212636 BANERJEE BURDWAN DIVISION DHAR, GHATAKPARA, (CS1991054 ) CHINSURAH TALUK, HOOGHLY, Dob- 12-01-1964 ,WEST BENGAL 712101 ,PHONE: 033 2680 2170 6 ABHIJIT 1991 EXECUTIVE DIRECTOR SHILPA BHAWAN,28,3, PODDAR 9874047447 MUKHOPADHYAY WBSIDC COURT, TIRETTI, KOLKATA, ontaranga.abhijit@g (CS1991058 ) WEST BENGAL 700012 mail.com Dob- 24-12-1963 7 SUJAY SARKAR 1991 DIRECTOR (HR) BIDYUT UNNAYAN BHAVAN 9434961715 (CS1991059 ) WBSEDCL ,3/C BLOCK -LA SECTOR III sujay_piyal@rediff Dob- 22-12-1968 ,SALT LAKE CITY KOL-98, PH- mail.com 23591917 8 LALITA 1991 SECRETARY KHADYA BHAWAN COMPLEX 9433273656 AGARWALA WEST BENGAL INFORMATION ,11A, MIRZA GHALIB ST. agarwalalalita@gma (CS1991060 ) COMMISSION JANBAZAR, TALTALA, il.com Dob- 10-10-1967 KOLKATA-700135 9 MD. -

SPSRC 194.Pdf
15. Concerned Superintendent of Tehatta SD Hospital / Nadia DH / /Balurghat DH / Gangarampur r> Multi Speciality Hospital / Vidyasagar SG Hospital / Bolpur SDH / Uluberia SD Hospital / Baghajatin SG Hospital / Baghajatin SG Hospital / Egra SD Hospital / Asansol District Hospital / Katwa SD Hospital for release the concerned MOs for training programme 16. Concerned BMOH - Amta Rural Hospital / Digha AK BPHC, Ramnagar 1 / Nijmaithuna PHC, Ramnagar 2 for release the concerned MOs for training programme 17. System co-ordinator. IT for web posting ~~1 Joint Dired6r, SPSRC Department of Health & Family Welfare Govt. of West Bengal Annexure I: Name of the MOs for training: SI. Place of Training Name of the MO Place of Posting District No. (lst Phase) 1. Dr. Tapas Malakar Tehatta SD Hospital Nadia RGKMCH Dr. Suparna 2. Nadia DH Nadia RGKMCH Mondal Dr. Prabir Kumar Dakshin 3. Balurghat OH RGKMCH Basak Dinajpur Khaspur RH (now Dakshin 4. Dr. Goutam Minji detailed at Balurghat RGKMCH Oinajpur DH) Dr. Koushik Gangarampur Multi Dakshin 5. RGKMCH Mandal Speciality Hospital Dinaipur Dr. Subhra Vidyasagar SG 6. South 24 pgs RGKMCH Bhattacharya Hospital 7. Dr. Biplab Pratihar Bolpur SDH Birbhum RGKMCH Dr. Amitava 8. Uluberia SD Hospital Howrah RGKMCH Sarkar MRBangur Dr. Dhirendra 9. Amta Rural Hospital Howrah Hospital under Kumar Gupta SSKMH MR Bangur Dr. Lakshmisona Vidyasagar SG 10. South 24 pgs Hospital under Auddy (Mallik) Hospital SSKMH MRBangur I 11. Dr. Debasis De Baghajatin se Hospital South 24 pgs Hospital under SSKMH MRBangur Dr. Siba Prasad 12. Baghajatin se Hospital South 24 pgs Hospital under Biswas SSKMH MRBangur 13. Dr. [yoti Biswas Baghajatin se Hospital South 24 pgs Hospital under SSKMH MRBangur Dr. -

Date Wise Details of Covid Vaccination Session Plan
Date wise details of Covid Vaccination session plan Name of the District: Darjeeling Dr Sanyukta Liu Name & Mobile no of the District Nodal Officer: Contact No of District Control Room: 8250237835 7001866136 Sl. Mobile No of CVC Adress of CVC site(name of hospital/ Type of vaccine to be used( Name of CVC Site Name of CVC Manager Remarks No Manager health centre, block/ ward/ village etc) Covishield/ Covaxine) 1 Darjeeling DH 1 Dr. Kumar Sariswal 9851937730 Darjeeling DH COVAXIN 2 Darjeeling DH 2 Dr. Kumar Sariswal 9851937730 Darjeeling DH COVISHIELD 3 Darjeeling UPCH Ghoom Dr. Kumar Sariswal 9851937730 Darjeeling UPCH Ghoom COVISHIELD 4 Kurseong SDH 1 Bijay Sinchury 7063071718 Kurseong SDH COVAXIN 5 Kurseong SDH 2 Bijay Sinchury 7063071718 Kurseong SDH COVISHIELD 6 Siliguri DH1 Koushik Roy 9851235672 Siliguri DH COVAXIN 7 SiliguriDH 2 Koushik Roy 9851235672 SiliguriDH COVISHIELD 8 NBMCH 1 (PSM) Goutam Das 9679230501 NBMCH COVAXIN 9 NBCMCH 2 Goutam Das 9679230501 NBCMCH COVISHIELD 10 Matigara BPHC 1 DR. Sohom Sen 9435389025 Matigara BPHC COVAXIN 11 Matigara BPHC 2 DR. Sohom Sen 9435389025 Matigara BPHC COVISHIELD 12 Kharibari RH 1 Dr. Alam 9804370580 Kharibari RH COVAXIN 13 Kharibari RH 2 Dr. Alam 9804370580 Kharibari RH COVISHIELD 14 Naxalbari RH 1 Dr.Kuntal Ghosh 9832159414 Naxalbari RH COVAXIN 15 Naxalbari RH 2 Dr.Kuntal Ghosh 9832159414 Naxalbari RH COVISHIELD 16 Phansidewa RH 1 Dr. Arunabha Das 7908844346 Phansidewa RH COVAXIN 17 Phansidewa RH 2 Dr. Arunabha Das 7908844346 Phansidewa RH COVISHIELD 18 Matri Sadan Dr. Sanjib Majumder 9434328017 Matri Sadan COVISHIELD 19 SMC UPHC7 1 Dr. Sanjib Majumder 9434328017 SMC UPHC7 COVAXIN 20 SMC UPHC7 2 Dr. -

Bus Route 1: from Thakurpukur 3A
Route 1 Route 1A ROUTE 2 ROUTE 2A ROUTE 3 2019-20 2019-20 2019-20 2019-20 2019-20 Bus Route 1: From Thakurpukur 3A Bus Route 1A: From Tollygunge Bus Route 2: From Bally Halt To Bus Route 2A: From Kamalgazi Bus Route 3: From Gariahat to Bus Stand to University P.S. to University University to University University Contact No: 9123786980 Contact No: 9123786980 Contact No: 9123786980 Contact No: 9123786980 Contact No: 9123786980 SL SL SL SL SL STOPPAGE STOPPAGE STOPPAGE STOPPAGE STOPPAGE NO NO NO NO NO SOUTHERN AVENUE POSTOFFICE 1 THAKURPUKUR 3A BUS STAND 1 1 RAMCHANDRAPUR 1 KAMALGAZI 1 GARIAHAT MORE SAROBAR CROSSING RASHBIHARI/SOUTHERN AVENUE 2 THAKURPUKUR BAZAR 2 2 BALLY HALT 2 GARIA DHALAI BRIDGE 2 DESHOPRIYO PARK CROSSING (OPP TOLLYGUNGE P.S.) 3 KADAMTALA/PORAASHATTALA 3 RABINDRA SAROBAR 3 BALLY GHAT 3 PATULI CROSSING 3 RASHBIHARI CROSSING 4 SILPARA 4 CHARU MARKET 4 DUNLOP 4 BAGHAJATIN 4 KALIGHAT TRAM DEPOT 5 SAKHERBAZAR 5 BHABANI CINEMA METRO 5 BONHOOGLY 5 HILAND PARK 5 HAZRA CROSSING 6 JANAKALYAN 6 TOLLYGUNGE PHARI 6 GHOSH PARA 6 AJAY NAGAR 6 PURNA CINEMA 7 BEHALA CHOWRASTHA 7 TIPU SULTAN MOSQUE 7 SINTHEE MORE 7 MUKUNDAPUR 7 JADU BAZAR (BHAWANIPUR METRO) 8 BLIND SCHOOL 8 DHAKA KALIBURI 8 CHIRIAMORE 8 METRO CASH AND CARRY 8 ELGIN ROAD 9 MANTON 9 NAVINA CINEMA 9 PAIKPARA BT ROAD 9 KALIKAPUR KHAL BRIDGE 9 EXIDE MORE (RABINDRA SADAN) 10 BEHALA TRAM DEPOT 10 LORDS MORE 10 TALA POST OFFICE 10 RUBY 10 LORDS SINNA ROAD 11 BEHALA PS 11 SOUTH CITY 11 SHYAM BAZAR 11 ULTADANGHA HUDCO 11 CAMAC ST. -

62/9, Haripada Dutta Lane, Tollygunge, Kolkata- 700033
JADAVPUR EXECUTIVES SRL NAME DESIGNATION D.O.B AREA TELE. NO(R) Residential address Year 1 ACHYUTANANDA MANDAL DY. AREA MANAGER 06.01.1947 JDP 2402-6973 33/B, NaskarPara Road, P.O- Paschimputiary, Kol-41 2007 /JDP 2 AJAY KUMAR CHAKRABARTI DE/JDV & AM/JDV 01.03.1950 JDV 2425-1425 7, Chittaranjan Park, Flat- B/1, Sankalpa Co-operative, Jadavpur, 2010 Kolkata- 700032 3 AJIT KUMAR DEBNATH AGM / ADMIN / JDV 06.01.1951 JDP 2477-6151 Vill-Manikpur Ghosalpara, P.O-Harinavi, 24 Pgs (S), Kolkata -700148. 2011 4 AMIT KUMAR GUPTA DGM / NWO-JDP,Offg. 02.12.1951 JDV 2346-4646 1/4, Rajendra Banerjee Road, Behala, Kolkata- 700034 2011 5 AMIYA DAS 103223 07.02.1951 JDP 2410-1234 E-32,Kalachand Para,Kamdahari,P.O- Garia, Kol -700084 2011 6 AMIYA SANKAR GUPTA SDE / OFFTG./RLU-21 01.12.1948 JDP 2473-9950 52/A, Bank Colony , P.O- Dhakuria, Kolkata- 700031 2008 7 ANIL CHANDRA BISWAS SDE/OFFTG./CR-II/JDP 02.01.1950 JDP 2431-2100 B/25, Baudipur Road, P.O- Bansdroni, Kolkata- 700070 2010 8 ARUN KUMAR BANERJEE OFFTG/ DE/ RKT / 03.01.1948 JDV 2428-6060 226/5/1, N S C Bose road, Flat No. -03, Kolkata- 700092 2008 EXTL 9 ARUN ROY CHOWDHURY SDE / OFFTG 24.04.1950 JDP 2462-6161 East Balia, Balia Main Road, P.O- Garia, Kolkata- 700084 2010 10 ASHIM KUMAR SENGUPTA J.T.O. 04.01.1949 JDP 2422-5500 62/9, Haripada Dutta Lane, Tollygunge, Kolkata- 2009 700033 11 ASIS KUMAR HALDAR 100847 09.04.1952 JDP 2436-5596 F/154, B. -

Bengal Ambuja Upohar Condoville - E M Bypa… About Dream Homes Project Optimizes Open Space to Ensure Maximum Comfort and Security
https://www.propertywala.com/bengal-ambuja-upohar-condoville-kolkata Bengal Ambuja Upohar Condoville - E M Bypa… about dream homes Project optimizes open space to ensure maximum comfort and security. The buildings are oriented to maximize light and air flow, creating a cheerful environment for healthy living. Project ID : J485271190 Builder: Bengal Ambuja Properties: Apartments / Flats, Independent Houses Location: Near Railway station, E M Bypass, Kolkata (West Bengal) Completion Date: Dec, 2009 Status: Completed Description Bengal Ambuja we nurture a dream...making a difference to the way people live in Kolkata. With Upohar- The Condoville at Chawkgaria, off EM Bypass, we are transforming this dream into reality. Spread over 18 acres of sylvan settings, Upohar~The Condo-ville will emerge as the next dream home for tomorrow’s Kolkata. Like our other projects, Upohar furthers the Bengal Ambuja mission ...Homes that are more than four walls...Home plus. Affordable for people from all walks of life. Which is why, Upohar~The Condo-ville will have Efficiency LIG, 248 one bedroom apartments, Comfort MIG, 360 two bedroom apartments and Luxury HIG, 605 two to five bedroom apartments including duplex and triplex. Upohar - The Condoville It optimizes open space to ensure maximum comfort and security. The buildings are oriented to maximize light and air flow, creating a cheerful environment for healthy living. South of Upohar are one Efficiency and two Comfort apartment buildings, ground plus seventeen. And up north are eleven Luxury apartment buildings, ground plus nineteen. Leaving the south and south-east open, for easy wind flow. The design emphasis is on creating large central open spaces, allowing Nature to form the core of Upohar. -
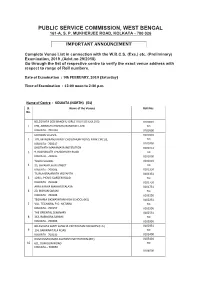
Complete Names and Addresses of the Venues of the Upcoming West
PUBLIC SERVICE COMMISSION, WEST BENGAL 161-A, S. P. MUKHERJEE ROAD, KOLKATA - 700 026 IMPORTANT ANNOUNCEMENT Complete Venue List in connection with the W.B.C.S. (Exe.) etc. (Preliminary) Examination, 2019 ,(Advt.no 29/2018) Go through the list of respective centre to verify the exact venue address with respect to range of Roll numbers. Date of Examination : 9th FEBRUARY, 2019 (Saturday) Time of Examination : 12:00 noon to 2:30 p.m. Name of Centre : KOLKATA (NORTH) (01) Sl. Name of the Venues Roll Nos. No. BELEGHATA DESHBANDHU GIRLS' HIGH SCHOOL (HS) 0100001 1 69B, ABINASH CHANDRA BANERJEE LANE TO KOLKATA - 700 010 0100500 MODERN SCHOOL 0100501 2 17B, MANORANJAN ROY CHOUDHURY ROAD, PARK CIRCUS, TO KOLKATA - 700017 0100750 BHUTNATH MAHAMAYA INSTITUTION 0100751 3 9, RADHANATH CHOWDHURY ROAD TO KOLKATA - 700015 0101000 TOWN SCHOOL 0101001 4 33, SHYAMPUKUR STREET TO KOLKATA - 700004 0101350 TILJALA BRAJANATH VIDYAPITH 0101351 5 129/1, PICNIC GARDEN ROAD TO KOLKATA - 700039 0101750 ARYA KANYA MAHAVIDYALAYA 0101751 6 20, BIDHAN SARANI TO KOLKATA - 700006 0102250 TEGHARIA SIKSHAYATAN HIGH SCHOOL (HS) 0102251 7 VILL. TEGHARIA, P.O. HATIARA TO KOLKATA - 700157 0102550 THE ORIENTAL SEMINARY 0102551 8 363, RABINDRA SARANI TO KOLKATA - 700006 0102950 BELEGHATA SANTI SANGHA VIDYAYATAN FOR BOYS (H.S.) 0102951 9 1/4, BARWARITALA ROAD TO KOLKATA - 700010 0103400 DUM DUM KUMAR ASUTOSH INSTITUTIOIN (BR.) 0103401 10 6/1, DUM DUM ROAD TO KOLKATA – 700030 0104000 KHANNA HIGH SCHOOL (H.S.) SUB-CENTRE 'A' 0104001 11 9, SHIVKUMAR KHANNA SARANI TO KOLKATA – 700015 0104350 KHANNA HIGH SCHOOL (H.S.) SUB-CENTRE 'B'; 0104351 12 9, SHIVKUMAR KHANNA SARANI TO KOLKATA - 700015 0104700 BELEGHATA SANTI SANGHA VIDYAYATAN FOR GIRLS 0104701 13 1/8, BAROWARITALA ROAD TO KOLKATA - 700010 0105250 BELEGHATA DESHBANDHU HIGH SCHOOL (BR.) 0105251 14 17/2E, BELEGHATA MAIN ROAD TO KOLKATA – 700010 0105700 BAGMARI MANIKTALA GOVT. -

Limnology and Productivity Status in Wetlands (Beels) of 24
Journal of Entomology and Zoology Studies 2017; 5(2): 664-668 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Limnology and productivity status in wetlands JEZS 2017; 5(2): 664-668 © 2017 JEZS (Beels) of 24- South Parganas district, West Bengal Received: 25-01-2017 Accepted: 26-02-2017 N Rishikanta Singh N Rishikanta Singh, SK Das, Sanjeev Kumar, Dibakar Bhakta, S Behera, Department of Fisheries Resource TS Nagesh and Anandamoy Mondal Management, Faculty of Fishery Sciences, West Bengal University of Animal and Fishery Sciences, Budherhat Road, Chakgaria, Panchasayar, Abstract Kolkata-700 094, West Bengal, India The environmental parameters such as water temperature, pH, dissolved oxygen, transparency, free carbon dioxide and plankton biomass were monitored during the present study period between July, 2011 SK Das and June, 2012 from Bhara Haripota beel in Bhamanghata, South 24 Parganas district of West Bengal. Department of Fisheries Resource Management, Faculty of Fishery Carp culture was practised in such beels and the physico-chemical parameters of water were found to be Sciences, West Bengal University of suitable for the growth of aquatic organisms. The water temperature varied from 20.6 C to 31.0 C, pH Animal and Fishery Sciences, Budherhat -1 Road, Chakgaria, Panchasayar, from 7.2 to 8.2, dissolved oxygen from 4.18 to 5.76 mg l , transparency from 18.6 to 26.5 cm and free -1 Kolkata-700 094, West Bengal, India carbon-dioxide from 0.46 to 1.52 mg l . The phytoplankton densities ranged from 1957 to 3652 organisms l-1 and zooplankton from 589 to 954 organisms l-1. -

Government of West Bengal Irrigation & Waterways Department
Government of West Bengal Irrigation & Waterways Department Jalasampad Bhavan(3RD Fl.); Salt Lake; Kol-91 No. 226-IE/3M-54/2019 Dated: 02.02.2021 ORDER The Governor has been pleased to accord permission in terms of Rule 5(2) of W.B.S.(Duties, Rights & Obligations of Govt. Employees) Rules,1980 read with No.1853-F(P) dt.2.3.12 in favour of Smt. Sanchari Bit, Junior Engineer, Central Design Office, Irrigation and Waterways Directorate to acquire a residential flat measuring 840 sq. ft. super built area at Flat No.- 1D, harmony, BI1 Baghajatin Park, 20 Chak Garia, P.O.- Panch sayer, Kolkata-Ol at appreciated cost of Rs.34,62,000.00(Rupees Thirty Four lakhs sixty two thousand) only inclusive of registration fee etc. The permission is hereby accorded subject to the condition that securing compliance of all statutory duties, liabilities and obligations as enshrined under the provisions of the West Bengal Apartment Ownership Act'1971 and/or West Bengal (Regulation of Promotion of Construction and Transfer by Promoters)Act,1983 and rules framed there under as amended time to time and also any other rules/statues that may be applied to her in this context will be her sole responsibility and Government in no way will be responsible for violation or deviation of non-observance of such rules. This order is issued subject to the condition that Smt. Bit will remain answerable to the Govt. if any of the furnished facts/documents is found to be false/ incorrect in later date. The said property should have been reflected in returning of declaration of asset. -

31 Krishnanagar 3 32 Bethuadahari 2 33 Plassey 2 34 Rejinagar 2 35
EASTERN RAILWAY NOTICE NO. CW/JTBS/Sep/2016 Sealdah, Dated: 08.09.2016 THIS IS NOT A RAILWAY JOB, IT IS LIKE AN AGENT. NAME of WORK; Railway administration invites application in prescribed format for appointment of JAN SADHARAN TICKET BOOKING SEWAK (JTBS) for 3 years, for issuance of computerized unreserved ticket through Unreserved Ticketing System (UTS) at nearby locations of following Stations. Sl. Station Name Sl. Station Name Sl. Station Name Nos. Proposed JTBS Nos. Proposed JTBS Nos. Proposed JTBS 1 Sealdah 10 39 Jiaganj 2 77 Ballyghat 2 2 Bidhannagar Road 5 40 Krishnapur 1 78 Park Circus 5 3 Dum Dum Jn. 5 41 Lalgola 1 79 Ballygunge 5 4 Belghoria 2 42 Dum Dum Cantt 2 80 Dhakuria 2 5 Agarpara 3 43 Durganagar 2 81 Jadavpur 2 6 Sodepore 3 44 Birati 3 82 Baghajatin 2 7 Khardah 3 45 Bisarpara Kodlia 2 83 New Garia 2 8 Titagarh 2 46 New Barrackpore 2 84 Garia 2 9 Barrackpore 3 47 Madhyamgram 2 85 Sonarpur 2 10 Palta 2 48 Hridaypur 2 86 Subhasgram 2 11 Ichhapore 2 49 Barasat 2 87 Mallikpur 2 12 Shyamnagar 2 50 Bamangachhi 2 88 Baruipur 2 13 Jagaddal 2 51 Duttapukur 2 89 Kalyanpur 2 14 Kankinara 2 52 Bira 2 90 Hotar 2 15 Naihati 3 53 Guma 2 91 Dhamuah 2 16 Halisahar 2 54 Ashoknagar Road 2 92 Magrahat 2 17 Kanchrapara 2 55 Habra 2 93 Sangrampur 2 18 Kalyani 2 56 Maslandapur 2 94 Deula 2 19 Kalyani Ghosh Para 2 57 Gobardanga 2 95 Netra 2 20 Kalyani Simanta 2 58 Thakurnagar 2 96 Diamond Harbour 2 21 Madanpur 2 59 Chandpara 2 97 Sasan Rd 2 22 Simurali 2 60 Bongaon 2 98 Dhap Dhapi 2 23 Palpara 2 61 Sondalia 2 99 Gocharan 2 24 Chakdah 3 62 Harua -

JADAVPUR UNIVERSITY CENTRAL LIBRARY Kolkata – 700 032 17.1
JADAVPUR UNIVERSITY CENTRAL LIBRARY Kolkata – 700 032 17.1.2019 It is notified that as per the tender process of Rate Contract (NIQ NO. JU/BT/001/2018, dated. 04/09/2018) approved by the Library Committee Meeting dated 10/10/2018, the following vendors are selected to supply print books to Jadavpur University with the accepted discount stated below: Enlisted Vendors with S.D. participated in Tender and agreed to supply: SLN VENDORS'NAME ADDRESS CATEGORIES DF BDDKS O. 1 Bharat Book Block-GD/15,SEC-111, Salt 1,2,5 Distributors Lake City, Kolkata-7000106. Ph.0336454 2 Lndica Publishers & 7/31,Ansari Road, Darya Ganj, 2,3,5 N71e8w3 /Delhi4007-3618180.002 , Distributors Pvt. Ltd. Ph.01123243027/23242328 3 Overseas Press(I) Pvt. Ltd. 2/4,Ansari Road, Darya Guange, 1,2,3,4,5 New Delhi-110002, Mob.9810012994,011- 43476444. ST 4 Prashant Book Agency 4263/A/3/1 Floor, Ansari 2,3,4,5,6 Road, Darya Ganj, New Delhi- 110002, Ph.01165398961, Mob.9818852025 5 Sahitya Bhawan 14/71,Hospital Road, Agra - 2,3 282003,UP, Mob.8697172389/7697742384 6 Techniz Books 4/12,Kalkaji Exten.,Opp.Of 2,3,4,5 International Nehru Place Kalkaji, New Delhi- 110020,Ph.01126284790/911 7 University Book 79,ChauraRasta,Jaipur- 2,3,4,5 House Pvt. Ltd. 302003,Rajasthan, Mob.9414046753 Enlisted Vendors with S.D. participated in Tender but incapable to supply: SLN VENDORS'NAME ADDRESS CATEGORIES OFBOOKS O. ST 1 ACADEMIA FE-6, IITMARKET (1 FLOOR), 1,2,3,4,5,6, E-Books KHARAGPUR- 721302,Mob.9830282376;7044 082376 2 ACADEMIC INDIA 50/38/14/lH,DHARMATALA 1,2,3,4,5,6 ROAD,KASBA,KOL-700042,Mob.