Final Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
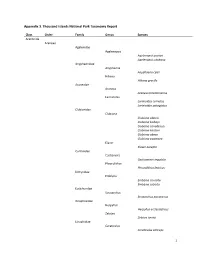
1 Appendix 3. Thousand Islands National Park Taxonomy Report
Appendix 3. Thousand Islands National Park Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis potteri Agelenopsis utahana Anyphaenidae Anyphaena Anyphaena celer Hibana Hibana gracilis Araneidae Araneus Araneus bicentenarius Larinioides Larinioides cornutus Larinioides patagiatus Clubionidae Clubiona Clubiona abboti Clubiona bishopi Clubiona canadensis Clubiona kastoni Clubiona obesa Clubiona pygmaea Elaver Elaver excepta Corinnidae Castianeira Castianeira cingulata Phrurolithus Phrurolithus festivus Dictynidae Emblyna Emblyna cruciata Emblyna sublata Eutichuridae Strotarchus Strotarchus piscatorius Gnaphosidae Herpyllus Herpyllus ecclesiasticus Zelotes Zelotes hentzi Linyphiidae Ceraticelus Ceraticelus atriceps 1 Collinsia Collinsia plumosa Erigone Erigone atra Hypselistes Hypselistes florens Microlinyphia Microlinyphia mandibulata Neriene Neriene radiata Soulgas Soulgas corticarius Spirembolus Lycosidae Pardosa Pardosa milvina Pardosa moesta Piratula Piratula canadensis Mimetidae Mimetus Mimetus notius Philodromidae Philodromus Philodromus peninsulanus Philodromus rufus vibrans Philodromus validus Philodromus vulgaris Thanatus Thanatus striatus Phrurolithidae Phrurotimpus Phrurotimpus borealis Pisauridae Dolomedes Dolomedes tenebrosus Dolomedes triton Pisaurina Pisaurina mira Salticidae Eris Eris militaris Hentzia Hentzia mitrata Naphrys Naphrys pulex Pelegrina Pelegrina proterva Tetragnathidae Tetragnatha 2 Tetragnatha caudata Tetragnatha shoshone Tetragnatha straminea Tetragnatha viridis -

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Butterflies and Moths of Dorchester County, Maryland, United States
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail -
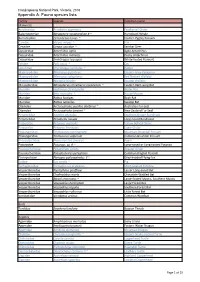
Report-VIC-Croajingolong National Park-Appendix A
Croajingolong National Park, Victoria, 2016 Appendix A: Fauna species lists Family Species Common name Mammals Acrobatidae Acrobates pygmaeus Feathertail Glider Balaenopteriae Megaptera novaeangliae # ~ Humpback Whale Burramyidae Cercartetus nanus ~ Eastern Pygmy Possum Canidae Vulpes vulpes ^ Fox Cervidae Cervus unicolor ^ Sambar Deer Dasyuridae Antechinus agilis Agile Antechinus Dasyuridae Antechinus mimetes Dusky Antechinus Dasyuridae Sminthopsis leucopus White-footed Dunnart Felidae Felis catus ^ Cat Leporidae Oryctolagus cuniculus ^ Rabbit Macropodidae Macropus giganteus Eastern Grey Kangaroo Macropodidae Macropus rufogriseus Red Necked Wallaby Macropodidae Wallabia bicolor Swamp Wallaby Miniopteridae Miniopterus schreibersii oceanensis ~ Eastern Bent-wing Bat Muridae Hydromys chrysogaster Water Rat Muridae Mus musculus ^ House Mouse Muridae Rattus fuscipes Bush Rat Muridae Rattus lutreolus Swamp Rat Otariidae Arctocephalus pusillus doriferus ~ Australian Fur-seal Otariidae Arctocephalus forsteri ~ New Zealand Fur Seal Peramelidae Isoodon obesulus Southern Brown Bandicoot Peramelidae Perameles nasuta Long-nosed Bandicoot Petauridae Petaurus australis Yellow Bellied Glider Petauridae Petaurus breviceps Sugar Glider Phalangeridae Trichosurus cunninghami Mountain Brushtail Possum Phalangeridae Trichosurus vulpecula Common Brushtail Possum Phascolarctidae Phascolarctos cinereus Koala Potoroidae Potorous sp. # ~ Long-nosed or Long-footed Potoroo Pseudocheiridae Petauroides volans Greater Glider Pseudocheiridae Pseudocheirus peregrinus -

CHECKLIST of WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea)
WISCONSIN ENTOMOLOGICAL SOCIETY SPECIAL PUBLICATION No. 6 JUNE 2018 CHECKLIST OF WISCONSIN MOTHS (Superfamilies Mimallonoidea, Drepanoidea, Lasiocampoidea, Bombycoidea, Geometroidea, and Noctuoidea) Leslie A. Ferge,1 George J. Balogh2 and Kyle E. Johnson3 ABSTRACT A total of 1284 species representing the thirteen families comprising the present checklist have been documented in Wisconsin, including 293 species of Geometridae, 252 species of Erebidae and 584 species of Noctuidae. Distributions are summarized using the six major natural divisions of Wisconsin; adult flight periods and statuses within the state are also reported. Examples of Wisconsin’s diverse native habitat types in each of the natural divisions have been systematically inventoried, and species associated with specialized habitats such as peatland, prairie, barrens and dunes are listed. INTRODUCTION This list is an updated version of the Wisconsin moth checklist by Ferge & Balogh (2000). A considerable amount of new information from has been accumulated in the 18 years since that initial publication. Over sixty species have been added, bringing the total to 1284 in the thirteen families comprising this checklist. These families are estimated to comprise approximately one-half of the state’s total moth fauna. Historical records of Wisconsin moths are relatively meager. Checklists including Wisconsin moths were compiled by Hoy (1883), Rauterberg (1900), Fernekes (1906) and Muttkowski (1907). Hoy's list was restricted to Racine County, the others to Milwaukee County. Records from these publications are of historical interest, but unfortunately few verifiable voucher specimens exist. Unverifiable identifications and minimal label data associated with older museum specimens limit the usefulness of this information. Covell (1970) compiled records of 222 Geometridae species, based on his examination of specimens representing at least 30 counties. -

Little Duarte's Pond Preserve Oak Bluffs, Tisbury and West Tisbury
Little Duarte’s Pond Preserve Oak Bluffs, Tisbury and West Tisbury Massachusetts Management Plan December 1, 2016 Approved by the Oak Bluffs and Tisbury Town Advisory Board () Approved by the Martha's Vineyard Land Bank Commission () Approved by Secretary Bowles of the Executive Office of Energy & Environmental Affairs () Julie Russell - Ecologist Maureen McManus Hill - Administrative Assistant Matthew Dix - Property Foreman Mary Tracy and Sean Terrill – Ecology Interns Executive Summary Little Duarte’s Pond Preserve comprises 6.5 acres of fee-simple land and 64 acres in agricultural preservation restriction. The preserve is named for the small fresh pond of which a portion is included in the preserve. Eighteenth-century deeds describe property boundaries extending into the center or through the pond. Historic deeds refer to the pond as “a little fresh pond” (1699), “a fresh pond” (1729, 1870), “the pond” (1776, 1892, 1899), “old house pond” (1869, 1896, 1913, 1933), “Lily Pond” (1869) and “Duart Ice Pond” (1939) for Antone Francise Duart who owned property on the pond and the Duart Farm off Old Pond Road in the 19th Century. The pond is labeled on a map created by Banks in the early 1900’s of the 18th century Nobnocket area as “Roach Pond” or “Duart Pond”. The spelling of the pond has changed to Duarte’s Pond over the years and later renamed Little Duarte’s Pond by the land bank’s Oak Bluffs town advisory board; outside of the historic references toward the pond, it will be written as Little Duarte’s Pond in this plan. The land surrounding Little Duarte’s Pond was used by native people as a village site for several thousand years. -

Foliage Insect Diversity in Dry Eucalypt Forests in Eastern Tasmania
Papers and Proceedings of the Royal Society of Tasmania, Volume 136, 2002 17 FOLIAGE INSECT DIVERSITY IN DRY EUCALYPT FORESTS IN EASTERN TASMANIA by H.J. Elliott, R. Bashford, S.J. Jarman and M.G. Neyland (with four tables, one text-figure and two appendices) ELLIOTT, H.]., BASHFORD, R., JARMAN,S.]' & NEYLAND, M.G., 2002 (3l:xii): Foliage insect diversity in dry eucalypt forests in eastern Tasmania. Papers and Proceedings ofthe Royal Society afTasmania 136: 17-34. ISSN 0080-4703. Forestry Tasmania, 79 Melville St., Hobart, Tasmania 7000, Australia. Species numbers and composition of the insect fauna occurring on trees and shrubs were studied in dry eucalypt forests in eastern Tasmania over nine years. In all, 1164 named and putative species representing 17 orders and 157 families were collected. The bulk of the species belonged to the orders Coleoptera (28%), Hymenoptera (25%), Hemiptera (18%), Lepidoptera (14%) and Diptera (10%). Of the species collected, 388 -- about one-third -- were identified at least to genus or species level. These included 21 named species not previously listed in the Tasmanian insect fauna and 90 undescribed species. A list of 22 host plants for 171 insect species was compiled from records of 132 insect species observed feeding during the study and from previous records ofinsect/host plant associations for 39 insect species found on the study plots. Most insects were feeding on eucalypts (127 insect species) and acacias (38 species). The most widely distributed and commonly collected species were several well-known pests ofeucalypts: Gonipterus scutellatus (Coleoptera: Curculionidae), Uraba lugens (Lepidoptera: N octuidae), Amorbus obscuricornis (Hemiptera: Coreidae), Chaetophyes compacta (Hemiptera: Machaerotidae) and Eriococcus coriaceous(Hemiptera: Eriococcidae). -

State Forest Environmental Assessment
INDIANA STATE FORESTS: ENVIRONMENTAL ASSESSMENT 2008 - 2027 Increased Emphasis on Management & Sustainability of Oak-Hickory Communitiesnities On the Indiana State Forest System DECEMBER 2008 Indiana Department of Natural Resources DIVISION OF FORESTRY ENVIRONMENTAL ASSESSMENT Increased Emphasis on Management and Sustainability of Oak-Hickory Communities On the Indiana State Forest System 2008-2027 DECEMBER 2008 Indiana Department of Natural Resources DIVISION OF FORESTRY INDIANA DEPARTMENT OF NATURAL RESOURCES The mission of the Indiana Department of Natural Resources is to serve as stewards of the natural, cultural, historic, and recreational resources for the benefit of present and future generations. STATE FOREST ENABLING LEGISLATION The legislation that provides the foundation for the management of state forests (IC 14-23-4-1) states: “It is the public policy of Indiana to protect and conserve the tim- ber, water resources, wildlife, and topsoil in the forests owned and operated by the divi- sion of forestry for the equal enjoyment and guaranteed use of future generations. How- ever, by the employment of good husbandry, timber that has a substantial commercial value may be removed in a manner that benefits the growth of saplings and other trees by thinnings, improvement cuttings, and harvest processes and at the same time provides a source of revenue to the state and counties and provides local markets with a further source of building material.” MISSION OF THE PROPERTY SECTION To manage, protect and conserve the timber, water, wildlife, soil and related forest re- sources for the use and enjoyment of present and future generations, and to demonstrate proper forest management to Indiana landowners. -

Great Lakes Entomologist
Vol. 34, No. 2 Fa II/Winter 2001 THE GREAT LAKES ENTOMOLOGIST PUBLISHED BY THE MICHIGAN ENTOMOLOGICAL SOCIETY THE GREAT LAKES ENTOMOLOGIST Published by the Michigan Entomological Society Volume 34 No.2 ISSN 0090-0222 TABLE OF CONTENTS Commentary on Ametropus species (Ephemeroptera: Amertropodidae) in North America W. P. McCafferty....................................................................................................... 1 First record ofAxymyiidae (Diptera: Nematocera: Axymyioidea) from Wisconsin Daniel K. Young and Anneke Lisberg ........................................................................ 7 Checklist of identified Lepidoptera collected at Mud Lake State Nature Preserve, Williams County, Ohio Roy W, Rings ............................................................................................................ 9 Dstribution of terrestrial isopods (Crustacea: Isopoda) throughout Michigan: early results Jennifer L. Stoyenoff ............................................................................................... 29 First Canadian record of Hexaco/a neoscatellae (Hymenoptera: Figitidae: Eucoilinae), a parasitoid of the shore fly, Scatella stagnalis Joshua C. Diamond, Vanessa A. Carney, Graeme D. Murphy, and Wayne R. Allen ...... 51 Hawkmoths (Lepidoptera: Sphingidae) collected in Grand Traverse and adjacent counties, Michigan Andrew W. Douglass, Christopher B. Douglass, and John F. Douglass .......................... 55 Second location for two rare Odonata in Ohio, Nannothemis bella and Ladona iulia, (Odonata: -

Plum Island Biodiversity Inventory
Plum Island Biodiversity Inventory New York Natural Heritage Program Plum Island Biodiversity Inventory Established in 1985, the New York Natural Heritage NY Natural Heritage also houses iMapInvasives, an Program (NYNHP) is a program of the State University of online tool for invasive species reporting and data New York College of Environmental Science and Forestry management. (SUNY ESF). Our mission is to facilitate conservation of NY Natural Heritage has developed two notable rare animals, rare plants, and significant ecosystems. We online resources: Conservation Guides include the accomplish this mission by combining thorough field biology, identification, habitat, and management of many inventories, scientific analyses, expert interpretation, and the of New York’s rare species and natural community most comprehensive database on New York's distinctive types; and NY Nature Explorer lists species and biodiversity to deliver the highest quality information for communities in a specified area of interest. natural resource planning, protection, and management. The program is an active participant in the The Program is funded by grants and contracts from NatureServe Network – an international network of government agencies whose missions involve natural biodiversity data centers overseen by a Washington D.C. resource management, private organizations involved in based non-profit organization. There are currently land protection and stewardship, and both government and Natural Heritage Programs or Conservation Data private organizations interested in advancing the Centers in all 50 states and several interstate regions. conservation of biodiversity. There are also 10 programs in Canada, and many NY Natural Heritage is housed within NYS DEC’s participating organizations across 12 Latin and South Division of Fish, Wildlife & Marine Resources. -

NATURAL RESOURCES COMMISSION Information Bulletin #2
Indiana Register NATURAL RESOURCES COMMISSION Information Bulletin #2 (Eighth Amendment) December 1, 2017 SUBJECT: Roster of Indiana Animals, Insects, and Plants that are Extirpated, Endangered, Threatened, or Rare (also described as Special Concern). I. HISTORY The initial roster was published February 1, 1992 (15 IR 848), republished April 1, 1991 (15 IR 1312); and subsequently amended to include additional species and published on February 1, 2005 (28 IR 1581). Since 2005, revisions have been made to several of the endangered species lists. The term "special concern" replaced the references to "rare" as it relates to wild animals and is expanded to include species in a legal status transition. Federal funding is available for species that are endangered or of special concern. In the fourth amendment, posted at 20070815-IR-312070469NRA on August 15, 2007, "rare", "threatened", and "extirpated" classifications for insect species were retained. Classification definitions were added for vascular plant species. In this document, species are reclassified and scientific names were modified. In the fifth amendment, posted at 20120125-IR-312120047NRA on January 25, 2012, the listing for endangered reptiles and amphibians was modified. Notable was removal of the American bald eagle from the endangered species list. Some species removed from the endangered list were redesignated as "special concern". Amendments were made to use scientific names that are consistent with those for species of animals listed in 312 IAC 9. In the sixth amendment, posted at 20140129-IR-312140023NRA on January 29, 2014, the listing for endangered birds, mollusks, insects, and vascular plants was modified. Notable was the removal of the peregrine falcon from the endangered species list. -

Butterflies and Moths of Bucks County, Pennsylvania, United States
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail