The 2Nd NO-Age Symposium on 'Genomic Instability in Human Brain'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Brief History of the DNA Repair Field Errol C Friedberg1
Errol C Friedberg npg Cell Research (2008) 18:-7. npg © 2008 IBCB, SIBS, CAS All rights reserved 1001-0602/08 $ 0.00 REVIEW www.nature.com/cr A brief history of the DNA repair field Errol C Friedberg1 1Laboratory of Molecular Pathology, Department of Pathology, University of Texas Southwestern Medical Center at Dallas, Dallas, TX, USA The history of the repair of damaged DNA can be traced to the mid-1930s. Since then multiple DNA repair mecha- nisms, as well as other biological responses to DNA damage, have been discovered and their regulation has been studied. This article briefly recounts the early history of this field. Keywords: DNA repair, biological responses to DNA damage, ultraviolet light, excision repair, enzymatic photoreactivation, mismatch repair, DNA damage tolerance, recombination Cell Research (2008) 18:3-7. doi: 10.1038/cr.2007.113; published online 24 December 2007 Introduction By the early 1940s it was becoming evident that agents that elicit mutational changes (such as ionizing and UV As pointed out in numerous reviews of the topic of DNA radiation) interact with and cause damage to the genetic repair [1], a full appreciation of the instability inherent in material of cells. Additionally, hints began to emerge that the chemistry of DNA and of its reactivity with a plethora living organisms can recover from the lethal effects of such of chemical and physical agents emerged surprisingly late damage [3]. These advances notwithstanding, “a combina- after the elucidation of the DNA structure by Crick and tion of intellectual biases and to a lesser extent political Watson. This surprise is reinforced by the realization that influences, constrained the emergence of DNA repair as an studies on the mutagenic effects of agents such as ionizing area of investigative inquiry in parallel with other aspects radiation and ultraviolet (UV) radiation date back to the of gene function. -
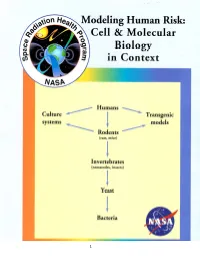
Animalexpreport1996.Pdf
1 Ernest Orlando Lawrence Berkeley National Laboratory University of California Berkeley, CA, 94720 A Report On ANIMAL EXPERIMENTATION at the FRONTIERS of MOLECULAR, CELLULAR and TISSUE RADIOBIOLOGY Select Panel Members Mina J. Bissell, Ph.D. (Chairman) Berkeley National Laboratory Huber R. Warner, Ph.D. (Executive Secretary) Berkeley National Laboratory Susan M. Berget, Ph.D. Baylor College of Medicine R. J. M. Fry, M.D. Oak Ridge National Laboratory Philip C. Hanawalt, Ph.D. Stanford University Arthur Kornberg, M.D. Stanford University Louise Lutze-Mann, Ph.D. University of California, SF Kenneth A. Souza, M.A. NASA-Ames Research Center Robert Ullrich, Ph.D. Univ. of Texas, Medical Branch Jan Vijg, Ph.D. Beth Israel Hospital, Harvard Univ. Aloke Chatterjee, Ph.D. (ex officio) Berkeley National Laboratory Date: October 7, 1996 version 2 Individuals other than Panel Members who have contributed written material to this report: Barcellos-Hoff, Mary Helen, LBNL Callahan, Paul, NASA - Ames Research Center Campisi, Judith, LBNL Joseph, James, Tufts University Rabin, Bernard, University of Maryland, Baltimore County Other individuals consulted: Alpen, Edward, LBNL Blakely, Eleanor, LBNL Brennan, Kathleen, LBNL Budinger, Thomas, LBNL Castro, Joseph, LBNL Cooper, Priscilla, LBNL Cox, Ann, USAF School of Aerospace Medicine Glickman, Barry, University of British Columbia Gold, Lois, LBNL Kennedy, Ann, University of Pennsylvania Kronenberg, Amy, LBNL Nelson, Gregory, Loma Linda Ness, Suzanne, California Biomedical Research Association Schild, David, LBNL Terazaghi-Howe, Margaret, ORNL 3 TABLE OF CONTENTS I. STATEMENT OF WORK II. EXECUTIVE SUMMARY III. INTRODUCTION IV. BACKGROUND: RADIATION A. RADIATION QUALITY IN SPACE 1. NATURE OF RADIATION ENVIRONMENT IN LOW EARTH ORBIT (LEO) 2. -
Expression of the P48 Xeroderma Pigmentosum Gene Is P53-Dependent and Is Involved in Global Genomic Repair
Proc. Natl. Acad. Sci. USA Vol. 96, pp. 424–428, January 1999 Biochemistry Expression of the p48 xeroderma pigmentosum gene is p53-dependent and is involved in global genomic repair BYUNG JOON HWANG*†,JAMES M. FORD†‡,PHILIP C. HANAWALT‡, AND GILBERT CHU*§ *Departments of Medicine and Biochemistry and ‡Department of Biological Sciences, Stanford University School of Medicine, Stanford, CA 94305-5115 Contributed by Philip Hanawalt, November 5, 1998 ABSTRACT In human cells, efficient global genomic re- MATERIALS AND METHODS pair of DNA damage induced by ultraviolet radiation requires the p53 tumor suppressor, but the mechanism has been Cell Lines. All cell lines were grown in DMEM supple- mented with 10% fetal bovine serum at 37°C in 5% CO2. WI38 unclear. The p48 gene is required for expression of an ultra- 2 violet radiation-damaged DNA binding activity and is dis- fetal lung primary human fibroblasts and XP2RO (DDB ) rupted by mutations in the subset of xeroderma pigmentosum primary human skin fibroblasts from an XPE individual were obtained from American Type Culture Collection (Rockville, group E cells that lack this activity. Here, we show that p48 1 mRNA levels strongly depend on basal p53 expression and MD). XP89TO (DDB ) primary human skin fibroblasts from an XPE individual were a gift from Stuart Linn (University of increase further after DNA damage in a p53-dependent man- 2y2 ner. Furthermore, like p532y2 cells, xeroderma pigmentosum California, Berkeley). The p53 (041 mut) human fibro- group E cells are deficient in global genomic repair. These blasts were isolated after spontaneous loss of the remaining results identify p48 as the link between p53 and the nucleotide wild-type copy of p53 from primary skin fibroblasts obtained excision repair apparatus. -

History of DNA Repair: Four Decades of Studies of DNA Repair at NIH and the First Twenty-Four Years of the DNA Repair Interest Group Kenneth H
History of DNA Repair: Four decades of studies of DNA Repair at NIH and The first twenty-four years of the DNA Repair Interest Group Kenneth H. Kraemer, M.D. Vilhelm A. Bohr, M.D., Ph.D. NCI, NIH, Bethesda, MD NIA, NIH, Baltimore, MD Update of: Bohr, V.A. and Kraemer, K.H.: The DNA Repair Interest Group: a global village DNA Repair 4: 405-406 (2005) (editorial) • Group began 1985 (Building 37 – Bohr/Kraemer) • Videoconferences began 1995 • Monthly videoconferences • 14 linked sites across US • 130+ lectures archived at http://videocast.nih.gov • e-mail list: >1400 subscribers worldwide • website To join send a request to [email protected] http://videocast.nih.gov/PastEvents.asp?c=5 14 Linked Sites in DNA Repair Interest Group Videoconferences - 2009 • NIH – Bethesda, MD; NIA, Baltimore, MD; NCI, Frederick, MD; NIEHS, Research Triangle Park, NC • National Labs – Lawrence Livermore, Livermore, CA; Brookhaven, Upton, NY • Universities- State University of New York, Stony Brook, NY; Univ of Kentucky, Lexington, KY; Univ of Michigan, Ann Arbor, MI; Univ of North Carolina, Chapel Hill, NC; Wake Forest Univ, Winston-Salem, NC; Oregon Health & Science Univ, Portland, OR; Univ of Pittsburgh, Pittsburgh, PA; Georgetown Univ, Washington, DC; M.D. Anderson, Smithville, TX http://videocast.nih.gov/PastEvents.asp?c=5 DNA Repair Interest Group 2009 Seminar Series Tom Misteli March 17 Spatial Genome Organization in the Formation of Translocations and DNA Repair Dan Yarosh, AGI Dermatics January 27 Intercellular Communication of DNA Damage its Repair DNA -

2 0 0 5 a N N U a L R E P O
2005 ANNUAL REPORT BURROUGHS WELLCOME FUND HISTORICAL TIMELINE 1950’s 1955 - BWF established as a corporate foundation in Tuckahoe, New York 1955 - William N. Creasy appointed first president and board chair 1959 - First advisory committee appointed, on clinical pharmacology 1959 - First competitive award program launched—Clinical Pharmacology Scholar Award program 1970’s 1970 - BWF moves to Research Triangle Park, North Carolina (with Burroughs Wellcome Co.) 1971 - Dr. George H. Hitchings becomes president 1974 - Iris B. Evans appointed first executive director 1978 - BWF grantmaking reaches $1 million annually 1979 - Toxicology Scholar Award program launched 1980’s 1981 - Martha Peck appointed executive director 1981 - Molecular Parasitology Scholar Award program launched 1983 - Scholar Award in Pharmacoepidemiology program launched 1985 - Immunopharmacology of Allergic Diseases Awards launched 1987 - Hitchings Awards for Innovative Methods in Drug Design and Discovery launched 1988 - First BWF newsletter (FOCUS) published 1988 - BWF President George H. Hitchings receives the Nobel Prize in Physiology or Medicine, along with Dr. Gertrude Elion and Sir James Black 1990’s 1990 - Dr. Howard J. Schaeffer appointed president 1991 - First female member appointed to the board Dr. Gertrude Elion 1991 - First non-Wellcome representative appointed to the board Dr. Samuel Katz 1993 - BWF receives $400 million endowment from The Wellcome Trust 1993 - BWF becomes independent private foundation 1994 - Dr. Enriqueta Bond, becomes first full-time president -

Princess Takamatsu Symposium on DNA Repair and Human Cancers
Meeting Report Cancer Research Princess Takamatsu Symposium on DNA Repair and Human Cancers Lawrence A. Loeb1,2 and Susumu Nishimura3 Abstract The 40th International Symposium of the Princess Takamatsu Cancer Research Fund, entitled “DNA Repair and Human Cancers,” was held on November 10–12, 2009 at Hotel Grand Palace, Tokyo, Japan. The meeting focused on the role of DNA repair in preventing mutations by endogenous and exogenous DNA damage and increasing the efficacy of chemotherapeutic agents by interfering with DNA repair. The 14 presentations by the speakers from the United States, four from the United Kingdom, one each from Italy, The Netherlands, and France, and 13 from Japan, covered most aspects of DNA repair, spanning DNA damage, molecular structures of repair enzymes, and clinical studies on inhibition of DNA repair processes. Extensive time was reserved for discussions with the active participation of the 150 invited Japanese scientists. The choice of a symposium on DNA repair in human cancers resulted in part from the excellent basic and clinical studies that have been carried out for many years in Japan, and the general lack of recognition versus the importance of DNA repair in understanding carcinogenesis. Cancer Res; 70(11); 4269–73. ©2010 AACR. Background for screening agents that target specific DNA repair processes. In short, considering the centrality of DNA repair in cancer The importance of DNA repair in preventing human prevention, it is apparent that there is insufficient research cancers was first catapulted into prominence by studies on that focuses on DNA repair and associated processes for rare genetic diseases that exhibit an exceptionally high the maintenance of genetic stability. -

Frontiers of Mutagenesis and DNA Repair: a Workshop
[CANCER RESEARCH 64, 3357–3360, May 1, 2004] Meeting Report Frontiers of Mutagenesis and DNA Repair: A Workshop Mark R. Kelley,1 Alan E. Tompkinson,2 Kandace J. Williams,3 Norman R. Drinkwater,4 and Victor A. Fung5 1Herman B. Wells Center for Pediatric Research, Cancer Research Institute, Indianapolis, Indiana; 2Department of Radiation Oncology, University of Maryland School of Medicine, Baltimore, Maryland; 3Department of Biochemistry & Molecular Biology, Medical College of Ohio, Toledo, Ohio; 4Department of Oncology, McArdle Laboratory for Cancer Research, University of Wisconsin-Madison, Madison, Wisconsin; and 5Center for Scientific Review, NIH, Bethesda, Maryland Abstract this active site of both polymerases is remarkably tolerant of muta- tions with the resultant amino acid changes generating different en- The Chemical Pathology Study Section, Center for Scientific Review, zymes with altered biochemical properties. The remarkably similar NIH hosted a 1-day workshop in Ventura, California, in the winter of mutations in each DNA Pol I may indicate evolutionary mechanisms 2003. There were 32 invited participants including invited speakers. The workshop consisted of three sessions and focused on recent developments of survival during altered environmental conditions. For a more de- in our understanding of the molecular mechanisms of mutagenesis and tailed examination of in vivo mutagenesis, a DNA Pol I-deficient E. DNA repair, namely, error-prone DNA polymerases, DNA repair models coli strain transformed with an error-prone version of Pol I was used. and DNA repair networks. An attractive feature of this approach is that in vivo mutations in saturated cultures can be targeted to a specific plasmid reporter gene Introduction and are expected to result only from activity of the error-prone Pol I. -

CURRICULUM VITAE JUSTIN COURCELLE Portland State University Department of Biology P.O
CURRICULUM VITAE JUSTIN COURCELLE Portland State University Department of Biology P.O. Box 751 Portland, Oregon 97207-0751 phone: (503) 725-3866 email: [email protected] Education Post-Doc 2000 Mutagenesis, University of Paris Ph.D. 1999 Cancer Biology, Stanford University B.S. 1992 Biology, University of Vermont Employment Asst, Assoc, Professor, Portland State University, 2005-present Asst Professor, Mississippi State University, 2000-2005 Postdoctoral Research with Miloslav Radman, University of Paris, 1999-2000 Dissertation Research with Philip Hanawalt, Stanford University, 1994-1999 Graduate Research with Michael Lieber, Stanford University, 1992-1994 Undergraduate Research with Susan Wallace, University of Vermont 1990-1992 Publications • Perera AV; Mendenhall JB; Courcelle CT; Courcelle J. (2016) Cho endonuclease functions during DNA interstrand crosslink repair in Escherichia coli. Journal of Bacteriology (in press). • Courcelle J; Wendel BM; Livingstone DD; Courcelle CT. (2015) RecBCD is required to complete chromosomal replication: Implications for double strand break frequencies and repair mechanisms. DNA Repair 32:86-95. • Wendel BM; Courcelle CT; Courcelle J. (2014) Completion of DNA Replication in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America 111:16454-9. • Jeiranian HA; Schalow BJ; Courcelle CT; Courcelle J. (2013) Fate of the replicsome following arrest by UV-Induced DNA Damage in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America 110:11421-6. • Courcelle CT; Landstrom AJ; Anderson B; Courcelle J. (2012) Cellular Characterization of the Primosome and Rep Helicase in Processing and Restoration of Replication following Arrest by UV-Induced DNA Damage in Escherichia coli. -

Chapter 23. DNA Nucleotide Excision Repair
1 Chapt 23 DNA nucleotide excision repair 210214at nciis-p001.nci.nih.gov/ Drugs Against Cancer: Stories of Discovery and the Quest for a Cure Kurt W. Kohn, MD, PhD Scientist Emeritus Laboratory of Molecular Pharmacology Developmental Therapeutics Branch National Cancer Institute Bethesda, Maryland [email protected] CHAPTER 23 DNA nucleotide excision repair (NER): cutting out the damage. Evolution has come up with an amazing set of tools to repair the great variety of damage that DNA can accrue. The repair tools can sustain life despite damage from radiation, environmental chemicals, chemotherapy, metabolic errors, reactive oxygen species from aerobic metabolism, cosmic rays, etc. The major sites of chemical damage to DNA, as understood in 1993, are shown in Figure 23.1. In view of these hazards, Tom Lindahl surmised that specific mechanisms must exist to repair those various kinds of damage to DNA (Lindahl, 1982). Lindahl shared the 2016 Nobel Prize in Chemistry with Aziz Sancar and Paul Modrich for their discoveries of DNA repair mechanisms (Figure 23.2) (Cleaver, 2016; Orren, 2016; Van Houten, 2016; Zagorski, 2005). The various DNA repair mechanisms are outlined in Figure 23.3, which also indicates in the legend the chapters that discuss each of them. The current chapter delves into the tools that repair the greatest variety of chemical damage to DNA: nucleotide excision repair (NER), which cuts out a damaged piece of DNA strand and replaces it with a good piece. There are two types of NER: global-NER and transcriptioncoupled NER (TCNER). Global-NER repairs DNA damage that may be present anywhere in the genome, whereas TCNER repairs DNA damage at sites where RNA polymerase is transcribing the genome. -

1 ENVIRONMENTAL MUTAGEN SOCIETY Thirty-Third Annual
ENVIRONMENTAL MUTAGEN SOCIETY Thirty-Third Annual Meeting Frontiers Beyond the Human Genome Hilton Hotel, Anchorage, Alaska April 27-May 2, 2002 The Environmental Mutagen Society was founded in 1969 and is incorpo- rated under the laws of the District of Columbia. Its purpose is to encour- age the study of mutagens in the human environment, particularly as they may affect public health, and to engage in and sponsor research and the dissemination of information related to mutagens. Membership is open to all interested scientists. OFFICERS President David M. DeMarini U.S. Environmental Protection Agency President Elect Lawrence A. Loeb University of Washington Past President James D. Tucker Lawrence Livermore National Laboratory Treasurer Michael J. Plewa University of Illinois at Urbana Secretary Jenness B. Majeska Boehringer Ingelheim Pharmaceuticals, Inc. Executive Director Tonia M. Masson EMS Headquarters 1767 Business Center Drive Suite 302 Reston, VA 20190 Phone: (703) 438-8220 Fax: (703) 438-3113 E-mail: [email protected] 1 COUNCILORS 2002-2005 William Au (2002) Daniel Benz (2004) John DeLuca (2002) John Essigmann (2004) Elizabeth George (2002) Peggy Guzzie (2004) Gerald Holmquist (2002) Kathleen Hill (2004) Barbara Shane (2002) Jennifer Sasaki (2004) Marilyn Aardema (2003) Stefano Bonassi (2005) Philip Hanawalt (2003) Lidia Cosentino (2005) Makoto Hayashi (2003) David Kirkland (2005) Suzanne Morris (2003) Mats Ljungman (2005) Martina Veigl (2003) Barbara Parsons (2005) PROGRAM COMMITTEE 2002 ANNUAL MEETING Chair: Lawrence A. Loeb Thomas A. Kunkel Marilyn J. Aardema Veronica M. Maher Richard J. Albertini William B. Mattes Bruce N. Ames Raymond J. Monnat William J. Bodell Leona D. Samson James E. -

DNA Repair: a Spatially Rooted Analysis of the Development of a Scientific Community Marion Maisonobe
DNA Repair: a spatially rooted analysis of the development of a scientific community Marion Maisonobe To cite this version: Marion Maisonobe. DNA Repair: a spatially rooted analysis of the development of a scientific commu- nity. 2nd Geography of Innovation International Conference, Utrecht University, Jan 2014, Utrecht, Netherlands. halshs-01272421 HAL Id: halshs-01272421 https://halshs.archives-ouvertes.fr/halshs-01272421 Submitted on 3 Nov 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. DNA Repair: a spatially rooted analysis of the development of a scientific community Marion MAISONOBE* *UMR LISST, Université de Toulouse, 5, allées Antonio-Machado, 31058 Toulouse Cedex9 1. Introduction Our aim is to track the emergence of the DNA Repair field (1964-1975) using both bibliometric data and historical sources (interviews, archives, historical accounts). We intend to focus on the geographical diffusion of the research specialty: how did the scientific interest for DNA Repair mechanisms spread at the worldwide level? What role the first scientists specialized in the field play in the diffusion process? In order to localize specialized teams, we follow the use of the keyword “DNA Repair” in scientific publications indexed in bibliometric databases. -

Master Disclaimer
Program Director's Report for the Office of Health and Environmental Research August 1996 Prepared for Office of Health and Environmental Research Office of Energy Research U.S. Department of Energy Prepared by Ernest Orlando Lawrence Berkeley National Laboratory Berkeley, California 94720 operated by University of California for U.S. Department of Energy under Contract No. DE-AC03-76SF00098 PUB-778 MASTER DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government Neither the United States Government nor any agency thereof, nor any of their employees, make any warranty, express or implied, or assumes any legal liabili- ty or responsibility for the accuracy, completeness, or usefulness of any information, appa- ratus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessar- ily state or reflect those of the United States Government or any agency thereof. DISCLAIMER Portions of this document may be illegible in electronic image products. Images are produced from the best available original document. Ill 1.0 OHER Program Summary 1.1 Director's Overview 1 Program Integration 5 Center for Environmental Biotechnology