Hydrogeochemical Framework of Groundwater Within the Asutifi-North
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Asutifi South District
ASUTIFI SOUTH DISTRICT Copyright © 2014 Ghana Statistical Service ii PREFACE AND ACKNOWLEDGEMENT No meaningful developmental activity can be undertaken without taking into account the characteristics of the population for whom the activity is targeted. The size of the population and its spatial distribution, growth and change over time, in addition to its socio-economic characteristics are all important in development planning. A population census is the most important source of data on the size, composition, growth and distribution of a country’s population at the national and sub-national levels. Data from the 2010 Population and Housing Census (PHC) will serve as reference for equitable distribution of national resources and government services, including the allocation of government funds among various regions, districts and other sub-national populations to education, health and other social services. The Ghana Statistical Service (GSS) is delighted to provide data users, especially the Metropolitan, Municipal and District Assemblies, with district-level analytical reports based on the 2010 PHC data to facilitate their planning and decision-making. The District Analytical Report for the Asutifi South District is one of the 216 district census reports aimed at making data available to planners and decision makers at the district level. In addition to presenting the district profile, the report discusses the social and economic dimensions of demographic variables and their implications for policy formulation, planning and interventions. The conclusions and recommendations drawn from the district report are expected to serve as a basis for improving the quality of life of Ghanaians through evidence- based decision-making, monitoring and evaluation of developmental goals and intervention programmes. -

Asunafo North Municipal Assembly 1.4 Key Issues/Challenges of the Municipal Assembly
TABLE OF CONTENTS Table 1.1 ESTABLISHMENT OF THE MUNICIPALITY ...................................... Error! Bookmark not defined. 1.2 POPULATION STRUCTURE ............................................................. Error! Bookmark not defined. 1.3 MUNICIPAL ECONOMY .................................................................. Error! Bookmark not defined. REPUBLIC OF GHANA 1.3.1 AGRICULTURE ........................................................................ Error! Bookmark not defined. 1.3.2 ENERGY .................................................................................. Error! Bookmark not defined. COMPOSITE BUDGET 1.3.3 TOURISM ................................................................................ Error! Bookmark not defined. 1.3.4 ENVIRONMENT ...................................................................... Error! Bookmark not defined. FOR 2018-2021 1.3.5 HEALTH .................................................................................. Error! Bookmark not defined. PROGRAMME BASED BUDGET ESTIMATES 1.3.6 EDUCATION ............................................................................ Error! Bookmark not defined. 1.3.7 WATER AND SANITATION ..................................................... Error! Bookmark not defined. FOR 2018 1.3.8 MARKET CENTRE.................................................................... Error! Bookmark not defined. 1.3.9 ROAD NETWORK ................................................................... Error! Bookmark not defined. ASUNAFO NORTH MUNICIPAL ASSEMBLY -

Asutifi North District Assembly
REPUBLIC OF GHANA THE COMPOSITE BUDGET OF THE ASUTIFI NORTH DISTRICT ASSEMBLY FOR THE 2016 FISCAL YEAR ASUTIFI NORTH DISTRICT ASSEMBLY NARRATIVE STATEMENT- 2016 COMPOSITE BUDGET 1.0 INTRODUCTION Section 92 of the Local Government Act, 1993, Act 462 envisages the implementation of the Composite Budget System under which the budget of department of the District Assembly would be integrated into the budget of the District Assembly. The District Composite Budgeting System would achieve the following amongst others: a. Ensure the public funds follow functions to give meaning to the transfer of staff transferred from the Civil Service to the Local Government Service. b. Establish an effective integrated budgeting system which supports intended goals, expectation and performance of government at the local levels, and c. Facilitate harmonized development and introduce fiscal prudence in the management of public funds at the MMDA level. Since 2011, Government has been directing all Metropolitan, Municipal and District Assemblies (MMDAs) to prepare for each fiscal year, Composite Budget which integrates departments under Schedule 1 of the Local Government (Department of the District Assemblies) (Commencement) Instrument, 2009, (LI 1961). This policy initiative is to upscale full implementation of fiscal decentralization and ensures that the utilization of all public resources at the level takes place in an efficient, effective, transparent and accountable manner for improved service delivery. The Composite Budget of the Asutifi North District Assembly for the 2016 fiscal year has been prepared from the 2016 Annual Action Plan lifted from the 2016-2018 Medium Term Development Plan which is aligned to the Ghana Shared Growth and Development Agenda (GSGDA II, 2014-2017). -

Report of the Commission of Inquiry Into the Creation of New Regions
COMMISSION OF INQUIRY REPORT OF THE COMMISSION OF INQUIRY INTO THE CREATION OF NEW REGIONS EQUITABLE DISTRIBUTION OF NATIONAL RESOURCES FOR BALANCED DEVELOPMENT PRESENTED TO HIS EXCELLENCY THE PRESIDENT OF THE REPUBLIC OF GHANA NANA ADDO DANKWA AKUFO-ADDO ON TUESDAY, 26TH DAY OF JUNE, 2018 COMMISSION OF INQUIRY INTO In case of reply, the CREATION OF NEW REGIONS number and date of this Tel: 0302-906404 Letter should be quoted Email: [email protected] Our Ref: Your Ref: REPUBLIC OF GHANA 26th June, 2018 H.E. President Nana Addo Dankwa Akufo-Addo President of the Republic of Ghana Jubilee House Accra Dear Mr. President, SUBMISSION OF THE REPORT OF THE COMMISSION OF INQUIRY INTO THE CREATION OF NEW REGIONS You appointed this Commission of Inquiry into the Creation of New Regions (Commission) on 19th October, 2017. The mandate of the Commission was to inquire into six petitions received from Brong-Ahafo, Northern, Volta and Western Regions demanding the creation of new regions. In furtherance of our mandate, the Commission embarked on broad consultations with all six petitioners and other stakeholders to arrive at its conclusions and recommendations. The Commission established substantial demand and need in all six areas from which the petitions emanated. On the basis of the foregoing, the Commission recommends the creation of six new regions out of the following regions: Brong-Ahafo; Northern; Volta and Western Regions. Mr. President, it is with great pleasure and honour that we forward to you, under the cover of this letter, our report titled: “Equitable Distribution of National Resources for Balanced Development”. -

Brong Ahafo Region
Fact sheet Brong Ahafo Region Rural and Small Towns Water Services September 2015 Area: 39,557 km2 This fact sheet gives an overview of rural and small towns water services in the Brong Ahafo Region. It Number of districts: 27 is the result of the 2014 service monitoring round executed by the Community Water and Sanitation Total population: 2,213,973 Agency (CWSA) in collaboration with Metropolitan, Municipal and District Assemblies (MMDAs). Wa- Rural population: 1,933,710 ter services have been assessed against the indicators set out in CWSA’s ‘Framework For Assessing And Urban population: 280,263 Monitoring Rural And Small Towns Water Supply Services In Ghana’, available at www.cwsa.gov.gh Water Supply Facilities and their Functionality Figure 1 gives an overview of the number and functionality of water supply facilities in rural areas and small towns in the Brong Ahafo region. Figure 2 shows that a good proportion of water facilities continue to function (Handpumps 58% and Pipe Scheme 89%). The most commonly used type of handpumps in the region are Afridev (57%) and Ghana Modified India Mark II (33%). There are as many as 212 Limited Mechanised Schemes in the Region. In addition, there are several small towns and small community piped schemes, as presented in Table 1. The Limited Mechanized Schemes are found mostly in urban com- munities, and seem to complement water supply from the other types of piped schemes. Some of the Limited Mechanised Schemes have household and institutional connections, contrary to the national guidelines. The largest community-managed piped scheme in the region is the Wenchi Small Town Water System, with a design population of 39,410. -

Asutifi North District Assembly
Table of Contents PART A: STRATEGIC OVERVIEW ..............................................4 2 ESTABLISHMENT OF THE DISTRICT ........................................................................ 4 4. VISION ................................................................................................................................... 4 5. MISSION................................................................................................................................ 4 6. GOAL ..................................................................................................................................... 4 REPUBLIC OF GHANA 7. CORE FUNCTIONS OF THE ASSEMBLY ..................................................................... 5 8. DISTRICT ECONOMY ........................................................................................................ 5 9. KEY ACHIEVEMENTS IN 2020 ................................................8 1. REVENUE AND EXPENDITURE PERFORMANCE ................................................ 14 COMPOSITE BUDGET 1. NMTDF POLICY OBJECTIVES AND COST ........................... 16 2. POLICY OUTCOME INDICATORS AND TARGETS ............................................... 18 PART B: BUDGET PROGRAMME SUMMARY .............................. 20 FOR 2021-2024 PROGRAMME 1: MANAGEMENT AND ADMINISTRATION ......................................... 20 PROGRAMME 2: INFRASTRUCTURE DELIVERY AND MANAGEMENT .................. 34 PROGRAMME 3: SOCIAL SERVICES DELIVERY ......................................................... -

Asunafo North Municipality
ASUNAFO NORTH MUNICIPALITY i Copyright © 2014 Ghana Statistical Service ii PREFACE AND ACKNOWLEDGEMENT No meaningful developmental activity can be undertaken without taking into account the characteristics of the population for whom the activity is targeted. The size of the population and its spatial distribution, growth and change over time, in addition to its socio-economic characteristics are all important in development planning. A population census is the most important source of data on the size, composition, growth and distribution of a country’s population at the national and sub-national levels. Data from the 2010 Population and Housing Census (PHC) will serve as reference for equitable distribution of national resources and government services, including the allocation of government funds among various regions, districts and other sub-national populations to education, health and other social services. The Ghana Statistical Service (GSS) is delighted to provide data users, especially the Metropolitan, Municipal and District Assemblies, with district-level analytical reports based on the 2010 PHC data to facilitate their planning and decision-making. The District Analytical Report for the Asunafo North Municipality is one of the 216 district census reports aimed at making data available to planners and decision makers at the district level. In addition to presenting the district profile, the report discusses the social and economic dimensions of demographic variables and their implications for policy formulation, planning and interventions. The conclusions and recommendations drawn from the district report are expected to serve as a basis for improving the quality of life of Ghanaians through evidence-based decision-making, monitoring and evaluation of developmental goals and intervention programmes. -

Environmental Impacts of Rock Blasting from Newmont Ahafo
OPEN ACCESS Freely available online s r Journal of Geology & Geophysics Research Article Environmental Impacts of Rock Blasting From Newmont Ahafo Mining In Asutifi North District, Ghana 1 2 1 1 1 3 Nyantakyi EK *, Muri M , Kuranchie FA , Agyei PA , Domfeh MK and Uba F 1Department of Civil and Environmental Engineering, University of Energy and Natural Resources, Ghana; 2Newmont Ahafo Mining, Kenyasi, Ahafo Region, Ghana;3Department of Mechanical and Manufacturing Engineering, University of Energy and Natural Resources, Ghana Retraction Note The article entitled “ Environmental Impacts of Rock Blasting From Newmont Ahafo Mining In Asutifi North District, Ghana ,” has been accepted for publication in the Journal Geology and Geophysics considering the statements provided in the article as personal opinion of the author which was found not having any conflict or biasness towards anything. As the article was a perspective one, information provided by the author was considered as an opinion to be expressed through publication. Publisher took decision to make the article online solely based on the reviewers suggestion which considered the article not but a personal opinion of the author. However, it is found that the author have some personal concerns and issues, therefore, being retracted from the journal. *Correspondence to: Nyantakyi EK, Department of Civil and Environmental Engineering, University of Energy and Natural Resources, Ghana, Tel: 233248480088, E-mail: [email protected] Received: June 01, 2020; Accepted: July 25, 2020; Published: August 01, 2020 Citation: Nyantakyi EK,Muri M, Kuranchie FA, Agyei PA,Domfeh MK and Uba F (2020) Environmental Impacts of Rock Blasting From Newmont Ahafo Mining In Asutifi North District, Ghana. -
To Download Ahafo Region List
PHONE_NU PRESENT_STATIO GRADE FULLNAME STAFF_ID PRESENT_STATION_ADDRESS PRESENT_STATION_DISTRICT MBER N_REGION ADII NICHOLAS KWARTENG 204209 0200725378 AKRODIE SAVIOUR PRIMARY SCHOOL P..O.BOX 28 AKRODIE ASUNAFO NORTH MUNICIPAL AHAFO ADII ZAKARIA ISSAH 198750 0242726421 AHAFOMAN SENIOR HIGH/TECHNICAL SCHOOL ASUNAFO NORTH MUNICIPAL AHAFO ADII KWAME KWARTENG MENSAH 132244 0243253810 BECHEM SCHOOL FOR THE DEAF, P.O.BOX 34, BECHEM TANO SOUTH MUNICIPAL AHAFO ADII ABIGAIL ANOKYE 627313 0245101238 TECHIMANTIA METHODIST JHS, P. O. BOX 42, TECHIMANTIA TANO SOUTH MUNICIPAL AHAFO ADII YUSSIF IDDRISS 596412 0509476228 IBRAHIMIA ISLAMIC 'A' PRIMARY SCHOOL. P. O. BOX 89, DUAYAW NKWANTA TANO NORTH MUNICIPAL AHAFO ADII AGYEMANG-DUAH NYAMEKYE 539406 0249659714 HWIDIEM SENIOR HIGH,P.O.BOX 10,HWIDIEM,AHAFO ASUTIFI SOUTH DISTRICT AHAFO ADII LYDIA SANGBONG 309848 0547906627 KODIWOHIA D/A PRIMARY SCHOOL ASUTIFI NORTH DISTRICT AHAFO ADII JOSEPHINE NKETSIAH 595348 0242913522 DWOMO R/C PRIMARY SCHOOL TANO SOUTH MUNICIPAL AHAFO ADII THEOPHILUS KYEREMEH 204219 0243734984 SAMUEL OTU PRESBY SHS, P. O. BOX 37, TECHIMANTIA TANO SOUTH MUNICIPAL AHAFO ADII JACOB ADAGBUNO ABONA 507238 0249728811 TANOSO R/C JHS SCHOOL, P. O. BOX 3 TANO NORTH MUNICIPAL AHAFO ADII BRIGHT GYAMFI DANQUAH 619312 0249247804 TANOSO PRESBY JHS, P. O. BOX 12 TANO NORTH MUNICIPAL AHAFO ADII THOMAS MENSAH BOATENG 220908 0246678182 NTOTROSO ST. LAWRENCE R/C JHS. BOX 18 ASUTIFI NORTH DISTRICT AHAFO ADII BENJAMIN KWANE ASAMOAH 467441 0553755394 REGIONAL EDUCATION OFFICE BOX BH 197, AHAFO, BECHEM TANO SOUTH MUNICIPAL AHAFO ADII MARY KYEREMAA KONADU 281433 0543322429 GOASO METHODIST PRIMARY ASUNAFO NORTH MUNICIPAL AHAFO ADII ACQUAH LAWRENCE 646809 0240439128 BOX 239 FAWOHOYEDEN-GAOSO ASUNAFO NORTH MUNICIPAL AHAFO ADII WILLIAMS MASUBIR ALHASSAN 3375445 0246307980 HWIDIEM SENIOR HIGH SCHOOL, ASUTIFI SOUTH DISTRICT AHAFO ADII ZENABU MAMUDU 847132 0242352676 T. -

(Oasl) Minetral Royalties Received and Disbursed 2018
OFFICE OF THE ADMINISTRATOR OF STOOL LANDS (OASL) MINETRAL ROYALTIES RECEIVED AND DISBURSED 2018 NAME OF GOVERNMENT AGENCY: OASL BONO, BONO EAST AND AHAFO REGION TAX PAYER NAME: TAXPAYER IDENTIFICATION NUMBER (TIN): SECTOR ( Minerals /Oil/ Gas): MINERALS Reporting template KARIKARI prepared by FORDJUOR [email protected] RECEIPTS FROM COMPANIES Amount: (In Actual Attach suporting document Type of Receipt Currency of Volume/in-kind CEDIS Volume/In-kind Value/Paid Received by the Ministry of Finance GHS Dividend Other Receipts Received by OASL (Regional Office) Date(s) Amount Utilization Ground rent- All the payment for Brong-Ahafo Region are done by NEWMONT. 03/02/2018 1,852,539.19 Disbursement by OASL to the District Assemblies Asutifi North District Assembly 03/08/2018 401,638.68 Asunafo North Municipal Assembly 03/08/2018 127,298.99 Tano North District Assembly 03/08/2018 304,765.19 Asutifi South District Assembly 03/08/2018 185,193.70 Disbursement by OASL to the Stools Kenyasi No. 1 03/08/2018 103,683.16 Kenyasi No.2 03/08/2018 103,683.16 Ntotroso 03/08/2018 103,683.16 Mim 03/08/2018 61,134.44 Duayaw-nkwanta 03/08/2018 65,545.33 Adrobaa 03/08/2018 22,383.39 Yamfo 03/08/2018 61,575.44 Susuanso 03/08/2018 41,669.71 Afrisipa 03/08/2018 17,878.56 Ayomso 03/08/2018 18,422.76 Hwdiem 03/08/2018 58,354.99 Nkasiem 03/08/2018 17,058.48 Techire *40,290.10 Goaso 03/08/2018 4,227.49 Golden Stool 03/08/2018 38,881.19 Ahafo South Divisional Council 03/08/2018 42,575.40 Akwaboa 03/12/2018 32593.96 Mineral Royalties 21/2/2018 1,155,960.13 19/4/2018 -
Page 1 "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "J "
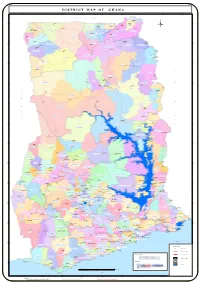
D I S T R I C T M A P O F G H A N A 3°0'0"W 2°30'0"W 2°0'0"W 1°30'0"W 1°0'0"W 0°30'0"W 0°0'0" 0°30'0"E 1°0'0"E B U R K I N A F A S O BAWKU MUNICIPAL Pusiga Bawku J" J" PUSIGA 11°0'0"N 11°0'0"N Gwollu Paga Binduri J" J" J" Zebilla BONGO J" BINDURI Bongo KASENA NANKANA WEST Navrongo KN WEST J" NANDOM Tumu J" Ü J" Garu LAMBUSSIE KARNI J" J" Nangodi J" J" SISSALA WEST Nandom Lambusie NABDAM BAWKU WEST BOLGATANGA GARU TEMPANE MUNICIPAL J" KASENA NANKANA Sandema Bolgatanga J" EAST Tongo BUILSA NORTH J" TALENSI Lawra J" LAWRA SISSALA EAST JIRAPA Jirapa Gambaga J" J" J" 10°30'0"N 10°30'0"N BUILSA SOUTH Bunkpurugu DAFFIAMA BUSSIE Fumbisi " MAMPRUSI EAST Daffiama J BUNKPURUGU YONYO J" WEST MAMPRUSI Nadawli J" J" Walewale Funsi NADOWLI-KALEO J" J" Yagaba MAMPRUGU MOAGDURI CHEREPONI Chereponi WA EAST J" Wa J" WA MUNICIPAL 10°0'0"N 10°0'0"N KARAGA GUSHIEGU Karaga J" J" Gushiegu R WA WEST Wenchiau J" E KUMBUMGU SABOBA P NORTH GONJA J" SAVELUGU NANTON Saboba Savelugu J" U Kumbungu TOLON J" J" B 9°30'0"N SAWLA/TUNA/KALBA Daboya YENDI MUNICIPAL 9°30'0"N Yendi Tolon SAGNERIGU Sagnarigu Sang J" J" J" J" L J" Tamale Tatale TAMALE NORTH SUB METRO MION J" I C Zabzugu Sawla J" J" O C TATALE T WEST GONJA ZABZUGU E Damongo J" Bole O J" 9°0'0"N 9°0'0"N NANUMBA NORTH F D' GONJA CENTRAL Bimbila J" I Buipe T V J" NANUMBA SOUTH BOLE O Wulensi O J" I G Salaga NKWANTA NORTH R J" Kpasa 8°30'0"N 8°30'0"N EAST GONJA Kpandai J" O E J" KPANDAI KINTAMPO NORTH NKWANTA SOUTH BANDA Nkwanta J" Yeji J" KRACHI NCHUMURU Banda Ahenkro J" Chindiri PRU J" Kintampo J" J" Dambai 8°0'0"N -

Implementing the Ahafo Benefit Agreements
IMPLEMENTING THE AHAFO BENEFIT AGREEMENTS: SEEKING MEANINGFUL COMMUNITY PARTICIPATION AT NEWMONT’S AHAFO GOLD MINE IN GHANA © 2018 by the University of British Columbia and Canadian International Resources and Development Institute (CIRDI). The material in this publication is copyrighted. Quoting, copying, and/or reproduc- ing portions or all of this work is permitted provided the following citation is used: Boakye, B., Cascadden, M., Kuschminder, J., Szoke-Burke, S., Werker, E. (2018). Implementing the Ahafo Benefit Agreements: Seeking Meaningful Community Participation at Newmont’s Ahafo Gold Mine in Ghana. Canadian International Resources and Development Institute (CIRDI) Report 2018-003 Program undertaken with the financial support of the Government of Canada provided through Global Affairs Canada. Programme réalisé avec l’appui financier du gouvernement du Canada agissant par l’en- tremise d’Affairs mondiales Canada. Canadian International Resources and Development Institute Vancouver, British Columbia, Canada www.cirdi.ca [email protected] Cover photo: Eric Werker i IMPLEMENTING THE AHAFO BENEFIT AGREEMENTS: SEEKING MEANINGFUL COMMUNITY PARTICIPATION AT NEWMONT’S AHAFO GOLD MINE IN GHANA June 2018 This report was written by Benjamin Boakye (Africa Centre for Energy Policy (ACEP)), Maggie Cascad- den (Simon Fraser University (SFU)), Jordon Kuschminder (Independent Social Performance (ISP)), Sam Szoke-Burke (Columbia Center on Sustainable Investment (CCSI)), and Eric Werker (Canadian International Resources and Development Institute (CIRDI) and Simon Fraser University (SFU)). Funding for the fieldwork was provided by CIRDI, and included in-kind contributions from ACEP, CCSI, ISP, and SFU. Fieldwork for the report was conducted in May 2017. We deeply thank the many people who spent time to discuss the implementation of the Ahafo benefit agreements with us.