Petition to List the Spring Pygmy Sunfish As Endangered Under the Endangered Species Act
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

U. S. Fish and Wildlife Service Environmental Action
U. S. FISH AND WILDLIFE SERVICE ENVIRONMENTAL ACTION STATEMENT FOR CATEGORICAL EXCLUSION Candidate Conservation Agreement with Assurances for the Spring Pygmy Sunfish AUGUST 2013 Within the spirit and intent of the Council on Environmental Quality's regulations for implementing the National Environmental Policy Act (NEPA), and other statutes, orders, and policies that protect fish and wildlife resources, I have established the following administrative record and determined that the following proposed action is categorically excluded from NEPA documentation requirements consistent with 40 CFR 1508.4, 516 DM 2.3A, 516 DM 2 Appendix 1, and 516 DM 6 Appendix 1.4. Proposed Action and Alternatives. The proposed action is the approval and implementation of a Candidate Conservation Agreement with Assurances (CCAA), proposed by Russ McDonald of Greenbriar Enterprises, L.L.C. to undertake management activities on his property to enhance, restore, or maintain habitat benefiting the spring pygmy sunfish. Candidate Conservation Agreements with Assurances, and the subsequent permits we may issue under section 10(a)(1)(A) of the Act (16 U.S.C. 1531 et seq.), encourage private and other non-Federal property owners to implement conservation efforts for target species, by assuring property owners that they will not be subjected to increased land use restrictions as a result of efforts to attract or increase the numbers or distribution of the species on their property, if that species becomes listed under the Act in the future. Application requirements and issuance criteria for permits through the Candidate Conservation Agreement with Assurances are found in the Code of Federal Regulations (CFR) at 50 CFR 17.22(d) and 17.32(d). -
Bluegill Diet Verification Project Outline Final W Letter of Support.Pdf
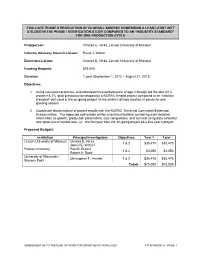
EVALUATE PHASE II PRODUCTION OF BLUEGILL SUNFISH COMPARING A LEAST-COST DIET UTILIZED IN THE PHASE I VERIFICATION STUDY COMPARED TO AN “INDUSTRY STANDARD” FOR ONE PRODUCTION CYCLE Chairperson: Charles E. Hicks, Lincoln University of Missouri Industry Advisory Council Liaison: Paula J. Moore Extension Liaison: Charles E. Hicks, Lincoln University of Missouri Funding Request: $75,000 Duration: 1 year (September 1, 2012 – August 31, 2013) Objectives: 1. Using consistent protocols, evaluate/determine performance of age-2 bluegill fed the diet (41% protein/<8.3% lipid) previously developed by a NCRAC funded project compared to an “industry standard” diet used in the on-going project at two distinct latitude location in ponds for one growing season. 2. Coordinate dissemination of project results with the NCRAC Technical Committee/Extension Subcommittee. The expected deliverable will be a technical bulletin containing such detailed information as growth, production parameters, size composition, and survival using data collected over grow out to market size; i.e., the first year from the on-going project plus this year’s project. Proposed Budgets: Institution Principal Investigators Objectives Year 1 Total Lincoln University of Missouri Charles E. Hicks 1 & 2 $35,475 $35,475 James E. Wetzel Purdue University Paul B. Brown 1 & 2 $3,050 $3,050 Robert A. Rode University of Wisconsin - Christopher F. Hartleb 1 & 2 $36,475 $36,475 Stevens Point Totals $75,000 $75,000 \AMENDMENT #2 TO THE PLAN OF WORK FOR GRANT #2010-38500-20929 ATTACHMENT A - PAGE 1 TABLE OF CONTENTS SUMMARY OVERVIEW (PARTICIPANTS, OBJECTIVES, AND PROPOSED BUDGETS) ....................... 1 JUSTIFICATION ........................................................................................................................................... 3 RELATED CURRENT AND PREVIOUS WORK ......................................................................................... -

Common Name: ALLIGATOR SNAPPING TURTLE
Common Name: BLUEBARRED PYGMY SUNFISH Scientific Name: Elassoma okatie Rohde and Arndt Other Commonly Used Names: none Previously Used Scientific Names: none Family: Elassomatidae Rarity Ranks: G2G3/S1S2 State Legal Status: Endangered Federal Legal Status: none Description: Like other pygmy sunfishes, the bluebarred pygmy sunfish is a small, laterally compressed species with a rounded caudal fin and single broad dorsal fin containing both spines and rays. It does not does exceed 30 mm standard length (1¼ inches). It has a small, surface- directed mouth and the top of its head is not scaled. It sides are typically marked with 8-14 (usually 10-12) dark vertical bars that are about 3 times wider than the lighter spaces between each bar. Breeding males are brilliantly colored with blue-green fins and blue-green flecks of pigment scattered over their dark black bodies. Females are light brown in color, but may be sparsely marked with yellow, green, or blue flecks of pigment on their bodies and fins. A male is pictured in the top photograph; the photo at the bottom of the account includes males and a female. Similar Species: In Georgia, the range of the bluebarred pygmy sunfish overlaps with two similar-appearing species of pygmy sunfish. In contrast to the bluebarred pygmy sunfish, the banded pygmy sunfish (Elassoma zonatum) has a post-ocular stripe and 1-3 dark blotches on their sides just above the pectoral fin (these blotches are usually visible in life but are much more evident in preserved specimens). The head of the everglades pygmy sunfish (Elassoma evergladei) has embedded scales and its body is mottled rather than barred. -

Mayan Cichlid (Cichlasoma Urophthalmum) Ecological Risk Screening Summary
U.S. Fish and Wildlife Service Mayan Cichlid (Cichlasoma urophthalmum) Ecological Risk Screening Summary Web Version – 11/01/2012 Photo: Alexander Calder 1 Native Range, and Status in the United States Native Range From Robins (2001): The Mayan cichlid is native to the Central American Atlantic slope waters of southeastern Mexico (including the Yucatán Peninsula), Belize, Guatemala, Honduras, and Nicaragua. Nonindigenous Occurrences From Schofield et al. (2011): “This species was first documented in Florida when specimens were observed and collected and observed in Everglades National Park in 1983; it is established in several areas in and around the park (Loftus 1987; Lorenz et al. 1997; Smith-Vaniz, personal communication [not cited]; Tilmant 1999) and Big Cypress National Preserve (Nico, unpublished data; Tilmant 1999).” “On the east side of Florida it has been recorded from Canal C-111 north to the Little River Canal (C-7 Canal) (Shafland 1995).” Cichlasoma urophthalmus Ecological Risk Screening Summary U.S. Fish and Wildlife Service – Web Version – 11/1/2012 “A single specimen was taken from a rock pit in Manatee County in October 1975 (Smith- Vaniz, personal communication [not cited]).” “Mayan ciclids have also been collected in Lake Okeechobee and Lake Osbourne, Palm Beach County in 2003 (Cocking 2003; Werner 2003).” “A new population was found in Charlotte Harbor in the summer of 2003 (Adams and Wolfe 2007; Associated Press 2003; Charlotte Harbor NEP 2004; Byrley, personal communication [not cited]). This is the most northern population known.” Reported established in Florida Panther National Wildlife Refuge (2005).” “A specimen was collected in Holiday Park in Broward County (International Game Fishing Association 2000).” “In 2006, this species was found to be established in Mobbly Bayou in Tampa Bay and in canals on Merritt Island in 2007 (Paperno et al. -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Fine Structure of the Retina of Black Bass, Micropterus Salmoides (Centrarchidae, Teleostei)
Histol Histopathol (1999) 14: 1053-1065 Histology and 001: 10.14670/HH-14.1053 Histopathology http://www.hh.um.es From Cell Biology to Tissue Engineering Fine structure of the retina of black bass, Micropterus salmoides (Centrarchidae, Teleostei) M. Garcia and J. de Juan Department of Biotechnology, University of Alicante, Alicante, Spain Summary. The structure of light- and dark-adapted 1968), 4) regular cone mosaics (Wagner, 1978), 5) the retina of the black bass, Micropterus salmoides has been existence of a well developed retinal tapetum in studied by light and electron microscopy. This retina nocturnal and bottom-living species (Wagner and Ali, lacks blood vessels at all levels. The optic fiber layer is 1978), 6) presence of foveae or areas with an increase in divided into fascicles by the processes of Muller cells photoreceptors and other neurons (Wagner, 1990), and 7) and the ganglion cell layer is represented by a single row a marked synaptic plasticity as is demonstrated by the of voluminous cells. The inner nuclear layer consists of formation of spinules (Wagner, 1980) during light two layers of horizontal cells and bipolar, amacrine and adaptation and their disappearance during dark interplexiform cells. In the outer plexiform layer we adaptation, and others. In summary, these features observed the synaptic terminals of photoreceptor celis, demonstrate the importance of vision in the mode of life rod spherules and cone pedicles and terminal processes and survival of the species. of bipolar and horizontal cells. The spherules have a Micropterus salmoides, known as black bass, is a single synaptic ribbon and the pedicles possess multiple member of the family Centrarchidae (Order Perciformes) synaptic ribbons. -

Winter Biology of Centrarchid Fishes C
Chapter 9 Winter biology of centrarchid fishes C. D. Suski and M. S. Ridgway 9.1 Introduction Temperate latitudes experience a predictable annual cycle of alternating warm and cold periods that can result in below freezing conditions, ice cover, and alterations to aquatic habitats that persist for a substantial portion of a year. Winter represents a very interesting and challenging time of the year that exerts a strong selective pressure on individual survival, community structure, and year class strength for centrarchid fishes. Despite the impact of this time on both individuals and populations, we are only beginning to comprehend how this period of the year can influence centrarchid fishes. The purpose of this chapter is to summarize the current literature that defines the ecological, behavioral, and physio- logical alterations experienced by centrarchid fishes both prior to and during winter. Because of the paucity of information on winter biology of centrarchid fishes, this chapter has been written in a general format whereby studies of different centrarchid fishes have been pooled to identify trends that exist across the entire family. Where appropriate, exceptions to these general trends have been noted. A general over-arching question does emerge from work to date despite the lack of broad research coverage in many areas of centrarchid winter biology: What physiological and ecological changes occur to ensure survival prior to and during a period of reduced energy intake? 9.2 Definition of “winter” We define “winter” as the period of the year between the autumnal equinox and prior to the onset of spawning in centrarchid fishes. -

Pennington Creek Fish
FAMILY: CENTRARCHIDAE (sunfishes) FAMILY: CYPRINIDAE (minnows) Bluegill Orangespotted Sunfish Smallmouth Bass Bigeye Shiner Lepomis macrochirus Lepomis humilis Micropterus dolomieu Notropis boops Characteristics: deep-bodied, small mouth, Characteristics: small with orange spots Characteristics: large mouth, vertical dark Characteristics: large eye relative to black spot posterior dorsal rays on side, long white-edged opercular flap bars are sometimes present on olive- body size, large mouth with a small bronze colored sides of the fish, juveniles head, dark lateral stripe extends from have an orange and black band on the cau- the lips through the eye to the end of dal fin the caudal peduncle Green Sunfish Redear Sunfish Largemouth Bass Blacktail Shiner Lepomis cyanellus Lepomis microlophus Micropterus salmoides Cyprinella venusta Characteristics: elongated body, large Characteristics: large, short opercular flap Characteristics: large mouth, upper jaw Characteristics: prominent black spot at mouth, black spot posterior dorsal & anal with a bright red crescent marking extends past the eye, dark midlateral the base of the caudal fin, large stripe from snout to base of the caudal fin diamond shaped scales outlined in black, breeding males develop yellow fins Longear Sunfish White & Black Crappie Spotted Bass Bluntnose Minnow Lepomis megalotis Pomoxis annularis, Pomoxis nigromacula- Micropterus punctulatus Pimephales notatus Characteristics: small, deep-bodied, long tus Characteristics: resembles the largemouth Characteristics: blunt, rounded -

History of Fishes - Structural Patterns and Trends in Diversification
History of fishes - Structural Patterns and Trends in Diversification AGNATHANS = Jawless • Class – Pteraspidomorphi • Class – Myxini?? (living) • Class – Cephalaspidomorphi – Osteostraci – Anaspidiformes – Petromyzontiformes (living) Major Groups of Agnathans • 1. Osteostracida 2. Anaspida 3. Pteraspidomorphida • Hagfish and Lamprey = traditionally together in cyclostomata Jaws = GNATHOSTOMES • Gnathostomes: the jawed fishes -good evidence for gnathostome monophyly. • 4 major groups of jawed vertebrates: Extinct Acanthodii and Placodermi (know) Living Chondrichthyes and Osteichthyes • Living Chondrichthyans - usually divided into Selachii or Elasmobranchi (sharks and rays) and Holocephali (chimeroids). • • Living Osteichthyans commonly regarded as forming two major groups ‑ – Actinopterygii – Ray finned fish – Sarcopterygii (coelacanths, lungfish, Tetrapods). • SARCOPTERYGII = Coelacanths + (Dipnoi = Lung-fish) + Rhipidistian (Osteolepimorphi) = Tetrapod Ancestors (Eusthenopteron) Close to tetrapods Lungfish - Dipnoi • Three genera, Africa+Australian+South American ACTINOPTERYGII Bichirs – Cladistia = POLYPTERIFORMES Notable exception = Cladistia – Polypterus (bichirs) - Represented by 10 FW species - tropical Africa and one species - Erpetoichthys calabaricus – reedfish. Highly aberrant Cladistia - numerous uniquely derived features – long, independent evolution: – Strange dorsal finlets, Series spiracular ossicles, Peculiar urohyal bone and parasphenoid • But retain # primitive Actinopterygian features = heavy ganoid scales (external -

Order Myctophiformes, Lanternfishes
Order Myctophiformes, lanternfishes • 241 species, 35 genera, 2 families • Deep sea pelagic and benthic, numerically dominant in deep sea habitats • Large terminal mouth (reminiscent of anchovy) • Adipose fin present • Compressed head and body (Myctophiformes = nose serpent shape) Lampridiformes • Large eyes Percopsiformes • Photophores Acanthomorpha •Hollow unsegmented spines on dorsal and anal fins •Rostal and premaxilla cartilidge and ligaments allow greater jaw protrusability Order Lampridiformes, opahs and oarfish Order Lampridiformes, opahs and oarfish • Oarfish • 19 species, 12 genera, 7 families – Longest teleost – over 30 feet • no true spines in fins – Only one individual observed • unique upper jaw protrusion – alive, used amiiform maxilla not directly attached to swimming ethmoid or palentine • deep bodied or ribbon-like • pelagic and deep water marine 1 Order Percopsiformes, trout perch, pirate perch, cavefish • 3 families, 7 genera, 9 species • All freshwater • Few with adipose fins – one of the most derived fishes with them • Pirate perch (Aphredoderidae) – One species – Fairly extensive parental care – Anus migration • Cavefish (Amblyopsidae) Zeiformes – Reduction or loss of eyes Gadiformes – Sensory papillae on head, body and tail Acanthomorpha •Hollow unsegmented spines on – Anus migration dorsal and anal fins •Rostal and premaxilla cartilidge – Convergent evolution of cave fish and ligaments allow greater jaw and other cave characins, protrusability catfishes etc. Order Zeiformes Order Gadiiformes • Dories • 555 species, -
![Kyfishid[1].Pdf](https://docslib.b-cdn.net/cover/2624/kyfishid-1-pdf-1462624.webp)
Kyfishid[1].Pdf
Kentucky Fishes Kentucky Department of Fish and Wildlife Resources Kentucky Fish & Wildlife’s Mission To conserve, protect and enhance Kentucky’s fish and wildlife resources and provide outstanding opportunities for hunting, fishing, trapping, boating, shooting sports, wildlife viewing, and related activities. Federal Aid Project funded by your purchase of fishing equipment and motor boat fuels Kentucky Department of Fish & Wildlife Resources #1 Sportsman’s Lane, Frankfort, KY 40601 1-800-858-1549 • fw.ky.gov Kentucky Fish & Wildlife’s Mission Kentucky Fishes by Matthew R. Thomas Fisheries Program Coordinator 2011 (Third edition, 2021) Kentucky Department of Fish & Wildlife Resources Division of Fisheries Cover paintings by Rick Hill • Publication design by Adrienne Yancy Preface entucky is home to a total of 245 native fish species with an additional 24 that have been introduced either intentionally (i.e., for sport) or accidentally. Within Kthe United States, Kentucky’s native freshwater fish diversity is exceeded only by Alabama and Tennessee. This high diversity of native fishes corresponds to an abun- dance of water bodies and wide variety of aquatic habitats across the state – from swift upland streams to large sluggish rivers, oxbow lakes, and wetlands. Approximately 25 species are most frequently caught by anglers either for sport or food. Many of these species occur in streams and rivers statewide, while several are routinely stocked in public and private water bodies across the state, especially ponds and reservoirs. The largest proportion of Kentucky’s fish fauna (80%) includes darters, minnows, suckers, madtoms, smaller sunfishes, and other groups (e.g., lam- preys) that are rarely seen by most people. -

Lepomis Spp. Technical Note Prepared by IUCN for the European Commission
Information on measures and related costs in relation to species considered for inclusion on the Union list This technical note has been drafted by a team of experts under the supervision of IUCN within the framework of the contract No 07.0202/2016/739524/SER/ENV.D.2 “Technical and Scientific support in relation to the Implementation of Regulation 1143/2014 on Invasive Alien Species”. The information and views set out in this note do not necessarily reflect the official opinion of the Commission. The Commission does not guarantee the accuracy of the data included in this note. Neither the Commission nor any person acting on the Commission’s behalf may be held responsible for the use which may be made of the information contained therein. Reproduction is authorised provided the source is acknowledged. This document shall be cited as: Zogaris, S.2017. Information on measures and related costs in relation to species considered for inclusion on the Union list: Lepomis spp. Technical note prepared by IUCN for the European Commission. This technical note provides information on the effectiveness of measures, alongside the required effort and resources, used to prevent the introduction, and to undertake early detection, rapid eradication, and management for the invasive alien species under review. Each table represents a separate measure. Date of completion: 04/12/2017 Comments which could support improvement of this document are welcome. Please send your comments by e-mail to [email protected] Species (scientific name) Genus: Lepomis (Rafinesque,