2004400 October 2004, Change Report and NCD Coding Policy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Treatment of Chyluria Secondary to Advanced Carcinoma of the Prostate
Prostate Cancer and Prostatic Diseases (2008) 11, 102–105 & 2008 Nature Publishing Group All rights reserved 1365-7852/08 $30.00 www.nature.com/pcan CASE REPORT The treatment of chyluria secondary to advanced carcinoma of the prostate SA Cluskey, A Myatt and MA Ferro Department of Urology, Huddersfield Royal Infirmary, Huddersfield, UK Chyluria has not been previously reported as being associated with carcinoma of the prostate. The most common cause is lymphatic filariasis, a parasitic disease. Non-parasitic chyluria is rare. We describe a case of chyluria associated with carcinoma of the prostate. We describe our successful management in this case and highlight how urologists may overcome problematic chyluria in patients with advanced carcinoma of the prostate. Prostate Cancer and Prostatic Diseases (2008) 11, 102–105; doi:10.1038/sj.pcan.4500994; published online 31 July 2007 Keywords: chyluria; androgen blockade; TURP Introduction 3-week history of back pain. He had not opened his bowels for 3 days but reported passage of flatus. He Chyluria occurs as a result of abnormal communication was referred to the acute surgical team for further in- between lymphatics and the urinary tract. It may be vestigation. associated with flank pain, fever, dysuria, haematuria At the time of admission, his symptoms included and chylous clot retention.1–3 Worldwide, the most general malaise, back pain and poor mobility. He was common cause is lymphatic filariasis, a mosquito-borne indeed bed bound. He reported no difficulty in passing parasitic disease endemic to tropical and subtropical urine, although he had been treated for a suspected areas. -

Chyluria in Pregnancy-A Decade of Experience in a Single Tertiary Care Hospital
Nephro Urol Mon. 2015 March; 7(2): e26309. DOI: 10.5812/numonthly.26309 Research Article Published online 2015 March 1. Chyluria in Pregnancy-A Decade of Experience in a Single Tertiary Care Hospital 1 1,* 1 1 2 Khalid Mahmood ; Ahsan Ahmad ; Kaushal Kumar ; Mahendra Singh ; Sangeeta Pankaj ; 2 Kalpana Singh 1Department of Urology, Indira Gandhi Institute of Medical Sciences, Patna, Bihar, India 2Department of Gynaecology, Indira Gandhi Institute of Medical Sciences, Patna, Bihar, India *Corresponding author : Ahsan Ahmad, Indira Gandhi Institute of Medical Sciences, Patna, Bihar, India. Tel: +91-9431457764, E-mail: [email protected] Received: ; Revised: ; Accepted: December 29, 2014 January 4, 2015 January 22, 2015 Background: Chyluria i.e. passage of chyle in urine, giving it milky appearance, is common in many parts of India but rare in west. Very few case of chyluria in pregnant female has been reported in literature. Persistence of this condition may have deleterious effects on health of child and mother. In the present study 43 cases of chyluria during pregnancy and their management seen over a period more than 10 years have been presented. Objectives: The study aims to present our experience of managing 43 cases with chyluria during pregnancy over a period of ten years from July 2003 to June 2014. Patients and Methods: Forty three pregnant patients with chyluria, who presented between July 2003 to June 2014 to the department of Urology, Indira Gandhi Institute of Medical Sciences, Patna were included. Patients underwent conservative management and/or sclerotherapy after evaluation. Follow-up of all patients was done by observation of urine color, routine examination of urine and test for post prandial chyle in urine up to 3 months after delivery. -

Non-Parasitic Chyluria: a Rare Experience
Chattogram Maa-O-Shishu Hospital Medical College Journal Volume 19, Issue 2, July 2020 Case Report Non-Parasitic Chyluria: A Rare Experience Faisal Ahmed1* Abstract Chyluria is the passage of chyle in the urine. The cause seems to be the rupture of 1 retroperitoneal lymphatics into the pyelocaliceal system, giving urine a milky Department of Paediatrics and Neonatology appearance. This condition if left untreated leads to significant morbidity because of Imperial Hospital Chattogram, Bangladesh. hematochyluria, recurrent renal colic, nutritional problems due to protein losses and immunosuppression resulting from lymphocyturia. Key words: Chyluria; Lymphatic; Pyelocaliceal system. INTRODUCTION Chyluria is the passage of chyle in the urine. The cause seems to be the rupture of retroperitoneal lymphatics into the pyelocaliceal system, giving urine a milky ap- pearance1-5. This communication is caused by the obstruction of lymphatic drainage proximal to intestinal lacteals, resulting in dilatation of distal lymphatics and the eventual rupture of lymphatic vessels into the urinary collecting system5-7. This con- dition if left untreated it leads to significant morbidity because of hematochyluria, recurrent renal colic, nutritional problems due to protein losses and immuno suppres- sion resulting from lymphocyturia. Various conservative measures like bed rest, high fluid intake, low-fat diet, fat-containing medium-chain triglycerides have been de- scribed. Chyluria may be classified as mild, moderate, or severe. Many sclerosing agents have been tried as silver nitrate, povidone iodine diluted in distillated water or pure. Povidone iodine with or without dextrose solution as a sclerosing agent was used successfully in a few studies. CASE REPORT A boy of 11 year and 6 month of age presented at OPD of Imperial Hospital, Chattogram on 9th August 2019, with the H/O passage of milky urine, mostly in the morning two years without any other complaint. -

“Chyluria” a Rare Isolated Manifestation of Filariasis Dr
DOI: 10.21276/sjams.2017.5.2.65 Scholars Journal of Applied Medical Sciences (SJAMS) ISSN 2320-6691 (Online) Sch. J. App. Med. Sci., 2017; 5(2E):632-634 ISSN 2347-954X (Print) ©Scholars Academic and Scientific Publisher (An International Publisher for Academic and Scientific Resources) www.saspublisher.com Case Report “Chyluria” A Rare Isolated Manifestation of Filariasis Dr. Tejaswini N1, Dr. Mrudul Ramachandran Nair2, Dr. Rekha N H3 1Post Graduate student, 2Post Graduate student, 3Associate Professor Department of General Medicine, Rajarajeswari Medical College and Hospital, Bangalore, Karnataka *Corresponding author Dr. Rekha N H Email: [email protected] Abstract: Chyluria is an uncommon condition characterized by passage of milky urine. Lymphatic filariasis is the most common cause of chyluria. Here we are reporting a case of chronic chyluria in an adult married female, diagnosed for filarial infection by W. bancrofti and treated medically with resolution of symptoms. Keywords: Chyluria, Filariasis, Wuchereria Bancrofti INTRODUCTION Urine routine revealed proteinuria +++ and on Chyluria is a rare clinical symptom due to microscopy there were no pus cells. Urine was negative passage of milky urine. Chyluria is a urological for AFB stain and urine culture did not show any manifestation of abnormal lymphatic system due to growth. Urinary triglyceride level was 400mg/dl and we retrograde or lateral flow of lymph from the lymphatics also observed clearance of milky white colour of urine of the kidney, ureter or bladder allowing chylous on adding equal amount of Ether and mixing vigorously material to be discharged into the urinary collecting (Figure 2). Midnight peripheral smear and DEC system. -

A Case Report of Chyluria with Proteinuria
2012 iMedPub Journals Vol. 3 No. 4:1 Our Site: http://www.imedpub.com/ ARCHIVES OF CLINICAL MICROBIOLOGY doi: 10.3823/255 Puranjay Saha1, Soma Sarkar1, Dipankar Sarkar2*, A Case Report Manideepa SenGupta1 of Chyluria with 1 Department of Microbiology, 2 Department of Critical-Care Correspondence: Medical College & Hospital, Medicine, Columbia-Asia Proteinuria - Filarial Kolkata, West-Bengal, India Hospital, Kolkata, [email protected] West-Bengal, India origin? An enigma * Dipankar Sarkar Department of Critical-Care Medicine, Columbia-Asia Hospital, Kolkata, West-Bengal, India Abstract Filarial infections are common in most tropical and subtropical regions of the world. We report a case of chyluria due to lymphourinary fistula in a filarial antigen negative case, the diagnosis of which was confirmed by the demonstration of microfilariae in urine as well as in peripheral blood only after diethyl carbamazine (DEC) provocation test. So In endemic areas, workup of filarial infection should be considered in a patient with chyluria even if the filarial antigen is non-reactive and microfilaria cannot be demonstrated initially. This article is available from: Keywords: Filaria, Chyluria, Filarial antigen www.acmicrob.com Introduction Filarial infections are common in most tropical and subtropical regions of the world. Numerically, the public health problem of lymphatic filariasis is greatest in India, China and Indone- sia [1]. Lymphatic filariasis is caused by Wuchereria bancrofti, Brugia malayi, or Brugia timori. The clinical manifestations are directly related to the occlusion of the lymphatic channels, thereby causing lymphangiectasia [2]. The most common pre- sentations of lymphatic filariasis are subclinical microfilaremia, acute adenolymphangitis, hydrocele ,and chronic lymphatic disease[3]. -

SNAPSHOT SNAPSHOT “Milky” Urine: a Case of Chyluria
SNAPSHOT SNAPSHOT “Milky” urine: a case of chyluria A 53-YEAR-OLD Asian man with type 2 diabetes mellitus presented to the Emergency Department with acute onset of 1: Oral fat tolerance test generalised muscle cramps. He reported a 2-month history of polydipsia, polyuria and passing “milky” urine with blood clots. He had travelled widely throughout subtropical Asia. On examination, he was normotensive, with no lymphaden- opathy, abdominal masses or oedema. Urinalysis showed marked proteinuria, glycosuria and haematuria.The Medical The Journal urine ofprotein Australia excretion ISSN: 0025-729X rate was 19 later confirmedJanuary to 2004 be 15.57180 2 89-89 g/24 h (reference interval [RI], 0.02– 0.15 g/24©The h). Medical Urine Journal triglyceride of Australia measurement 2004 www.mja.com.au and lipopro- tein Snapshotelectrophoresis confirmed the appearance of chylo- microns in the urine after an oral fat tolerance test (Box 1). Biochemical analysis of serum showed the following levels: sodium 121mmol/L (RI, 134–146mmol/L), potassium 4.6mmol/L (RI, 3.4–5.3mmol/L), creatinine 46µmol/L (RI, Urine of patient before a 75 g oral fat tolerance test (0 h) and at 60–105µmol/L), glucose 19.7mmol/L (RI, <5.5mmol/L), fer- serial time points (0.5, 1, 2, 3, 4, and 5 h) after the test. ritin 16 mg/L (RI, 30–620mg/L), 25-hydroxyvitamin D 11nmol/L (RI, >50 nmol/L), total cholesterol 5.2mmol/L (RI, <5.5mmol/L), and triglyceride 1.8mmol/L (RI, <1.8mmol/L). 2: Contrast lymphangiography The patient had marked hypoproteinaemia and hypoalbumin- aemia, with a total protein level of 39g/L (RI, 63–80g/L) and albumin level of 21g/L (RI, 35–50g/L), respectively. -
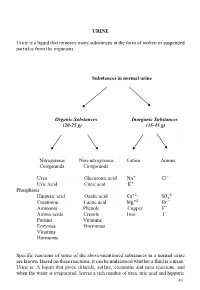
URINE Urine Is a Liquid That Removes Many Substances in the Form Of
URINE Urine is a liquid that removes many substances in the form of molten or suspended particles from the organism. Substances in normal urine Organic Substances Inorganic Substances (20-25 g) (35-45 g) Nitrogenous Non-nitrogenous Cation Anions Compounds Compounds Urea Glucuronic acid Na+ CI− Uric Acid Citric acid K+ Phosphates +2 −2 Hippuric acid Oxalic acid Ca SO4 Creatinine Lactic acid Mg+2 Br− Ammonia Phenols Copper F− Amino acids Cresols Iron I− Purines Vitamins Enzymes Hormones Vitamins Hormones Specific reactions of some of the above-mentioned substances in a normal urine are known. Based on these reactions, it can be understood whether a fluid is a urine. Urine is: A liquid that gives chloride, sulfate, creatinine and urea reactions, and when the water is evaporated, leaves a rich residue of urea, uric acid and hippuric 49 acid. Understanding whether a liquid is urine or not: •Determination of chloride, sulfate, urea, creatinine in liquid •Search for urea in the residual after the liquid has been evaporated Experiments will be carried out by sampling 2-3 ml into the tube. 1-Determination of chloride Liquid + a few drops of concentrated HNO3+ 2-3 drops of 5% AgNO3→ AgCl ↓ (white sediment) Nitric acid (HNO3 prevents the precipitation of phosphate and carbonate which could be precipitated by silver nitrate (AgNO3). 2-Determination of sulphate Liquid + several drops of 10% HCl + 2-3 drops BaCl2→ BaSO4↓ (white sediment) 3-Determination of urea Searched by two experiments: • Sodium hypobromite (NaOBr) • Using the enzyme urease a- Sodium -

1 Evaluation of Renal Disease
Manual of Pediatric Nephrology Kishore Phadke • Paul Goodyer Martin Bitzan Editors Manual of Pediatric Nephrology Editors Kishore Phadke Martin Bitzan Department of Pediatric Nephrology Division of Pediatric Nephrology Children’s Kidney Care Center Montreal Children’s Hospital St. John’s Medical College Hospital McGill University Bangalore, KA Montreal , QC India Canada Paul Goodyer Division of Pediatric Nephrology Montreal Children’s Hospital McGill University Montreal, QC Canada ISBN 978-3-642-12482-2 ISBN 978-3-642-12483-9 (eBook) DOI 10.1007/978-3-642-12483-9 Springer Heidelberg New York Dordrecht London Library of Congress Control Number: 2013948392 © Springer-Verlag Berlin Heidelberg 2014 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, speci fi cally the rights of translation, reprinting, reuse of illustrations, recita- tion, broadcasting, reproduction on micro fi lms or in any other physical way, and transmission or infor- mation storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. Exempted from this legal reservation are brief excerpts in connection with reviews or scholarly analysis or material supplied speci fi cally for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. Duplication of this publication or parts thereof is permitted only under the provisions of the Copyright Law of the Publisher’s location, in its current version, and permission for use must always be obtained from Springer. Permissions for use may be obtained through RightsLink at the Copyright Clearance Center. -

Urinalysis Cloudy/Milky Phosphaturia (Commonest – Crystal Precipitate In
Urinalysis Urinalysis Cloudy/milky Phosphaturia (commonest – crystal precipitate in high pH) Pyuria Chyluria Red Hematuria Hemoglobinuria/myoglobinuria Anthrocyanin in beets and blackberries Chronic lead and mercury poisoning Phenolphthalein (in bowel evacuants) Phenothiazines Rifampin Orange Dehydration Phenazopyridine (Pyridium) Sulfasalazine (Azulfidine) Yellow Normal Phenacetin Riboflavin Green-blue Biliverdin Indicanuria (tryptophan indole metabolites) Amitriptyline (Elavil) Indigo carmine Methylene blue Phenois (e.g., IV cimetidine [Tagamet], IV promethazine [Phenergan]) Resorcinol Triamterene (Dyrenium) Brown Urobilinogen Porphyria Aloe, fava beans, and rhubarb Chloroquine and primaquine Furazolidone (Furoxone) Metronidazole (Flagyl) Nitrofurantoin (Furadantin) Brown-black Alcaptonuria (homogentisic acid) Hemorrhage Melanin Tyrosinosis (hydroxyphenylpyruvic acid) Cascara, senna (laxatives) Methocarbamol (Robaxin) Methyldopa (Aldomet) Sorbitol Tom Walton January 2011 1 Urinalysis Variable Normal value High Low Specific gravity* 1.001 – 1.035 >1.020 <1.008 Dehydration Overhydration Diuretics Impaired conc. DM DI Urine osmolality 50 – 1200 mosm/l As above As above pH 5.5 – 6.5 >6.5 <5.5 Proteus Cystinuria RTA 1 and 2 Uricosuria Protein < 20 mg/dl * can be measured on dipstick/ osmolality cannot Haematuria > 3RBCs/hpf suggestive of significant haematuria on microscopy Dipstick testing Detects haematuria, haemoglobinuria and myoglobinuria Cells lysed on contact with reagent strip. Peroxidase activity of haemoglobin/ myoglobin utilised -

Urinalysis: a Comprehensive Review JEFF A
Urinalysis: A Comprehensive Review JEFF A. SIMERVILLE, M.D., WILLIAM C. MAXTED, M.D., and JOHN L PAHIRA, M.D. Georgetown Utiiversity School of Medicine, Washington, D.C. A complete urinalysis includes physical, chemical, and microscopic examinations. Midstream clean collection is acceptable in most situations, but tbe specimen sbould be examined within two hours of collection. Cloudy urine often is a result of precipitated phosphate crystals in alka- line urine, but pyuria also can be tbe cause. A strong odor may be the resuh of a concentrated specimen rather than a urinary tract infection. Dipstick urinalysis is convenient, but false-posi- tive and false-negative results can occur. Specific gravity provides a reliable assessment of the patient's hydration status. Microhematuria has a range of causes, from benign to life tbreatening. Glomerular, renal, and urologic causes of microbematuria often can be differentiated by otber elements of the urinalysis. Although transient proteijiuria typically is a henign condition, persis- tent proteinuria requires further work-up. Uncomplicated urinary tract infections diagnosed by positive leukocyte esterase and nitrite tests can be treated without culture. {Am Fam Physician 2005;71:1153-62. Copyright© 2005 American Academy of Family Physicians.) Seepage 1046 for rinalysis is invaluable in the ors (Table 1).^ Cloudy urine often is a result strength-of-fecommenda- diagnosis of urologic conditions of precipitated phosphate crystals in alkaline tion labels. such as calculi, urinary tract urine, but pyuria also can be the cause. Uinfection (UTI), and malig- The normal odor of urine is described as nancy. It also can alert the physician to the urinoid; this odor can be strong in concen- presence of systemic disease affecting the trated specimens but does not imply infec- kidneys. -

Analysis of Case Series of Milky Urine
[Downloaded free from http://www.urologyannals.com on Tuesday, November 18, 2014, IP: 197.132.255.244] || Click here to download free Android application for this journal Original Article Analysis of case series of milky urine: A single center and departmental clinical experience with emphasis on management perspectives: A prospective observational study Sham Sunder, Rajesh Jayaraman, Himanshu Sekhar Mahapatra, Satyanand Sathi, K. Venkataramanan, K. Prabhu, Anurag Gupta, Suman Sethi Department of Nephrology, Post Graduate Institute of Medical Education and Research, Dr. Ram Manohar Lohia Hospital, New Delhi, India Abstract Background: Milky urine can be due to chyluria or lipiduria due to nephrotic syndrome. Filarial chyluria usually responds to medical management while non-filarial cases may require surgical intervention. Aim: To perform a prospective observational study in patients presenting with milky urine in our centre over a period of one year from July 2011 to June 2012, a complete biochemical work up and imaging to find out the site of leakage of lymph if it is a case of chyluria, its response to medical management and the requirement of surgical intervention. Materials and Methods: Routine blood and urine investigations, 24 hour urine protein excretion, USG abdomen, serum lipid profile and rapid filarial antigen test were done in all. MRI abdomen was done in affordable patients. Renal biopsy was done in some chyluria patients for academic purpose and in milky urine with negative urine ether test. Sclerotherapy was done with 50% dextrose and 0.2% povidone iodine. Patients were followed up with 24 hour urine protein and triglyceride estimation. Results: 18 cases of milky urine were encountered. -

The Role of Urine Investigations in Urology Practice
Open Journal of Orthopedics, 2015, 5, 90-99 Published Online April 2015 in SciRes. http://www.scirp.org/journal/ojo http://dx.doi.org/10.4236/ojo.2015.54012 The Role of Urine Investigations in Urology Practice Muhammad Ujudud Musa Urology Unit, Department of Surgery, Federal Medical Centre Katsina, Katsina, Nigeria Email: [email protected] Received 6 February 2015; accepted 9 April 2015; published 14 April 2015 Copyright © 2015 by author and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Urine examination is one of the important armamentarium in the practice of urology and it is a fundamental test that is usually carried out for urology patients, it can be as simple as dipstick analysis to a complex hormonal assays. Urine examination is done worldwide as an extension of physical examination which provides a lot of information about the pathology, and both physical and chemical analysis of urine are highly informative. There are several types of urine investiga- tions, some of which include urinalysis, urine microscopy culture and sensitivity, urine micros- copy for ova or cyst of parasites, urine cytology, urine tumour antigens assays, urine hormonal assays, urine toxicology, urine quantitative measurement and urine acid fast bacilli. Uses of urine examinations in urology could be diagnostic, such as renal function test, evaluation of heamaturia, stone diseases, urinary tract infections, urologic cancers and infertility or monitoring and for prognosis. Uses of urine investigations in practice of urology cannot be over emphasized as it has many revealing information of the physiology and pathology of urologic organs.