Determination of Wing Cell Fate by the Escargot and Snail Genes in Drosophila
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Live Imaging of Drosophila Imaginal Disc Development
Live imaging of Drosophila imaginal disc development Silvia Aldaz1, Luis M. Escudero1,2, and Matthew Freeman3 Medical Research Council, Laboratory of Molecular Biology, Cambridge CB2 0QH, United Kingdom Communicated by A. Garcia-Bellido, Universidad Autonoma de Madrid, Madrid, Spain, June 25, 2010 (received for review March 19, 2010) Live imaging has revolutionized the analysis of developmental peripodial epithelium (PE), the latter contributing little to the biology over the last few years. The ability to track in real time the final adult structure; together they form a bag-like double epi- dynamic processes that occur at tissue and cellular levels gives thelium (Fig. 1A). During metamorphosis the imaginal discs are a much clearer view of development, and allows greater temporal completely remodeled in an evagination process comprising two resolution, than is possible with fixed tissue. Drosophila imaginal discrete stages: elongation, during which the columnar epithe- discs are a particularly important model of many aspects of de- lium lengthens and changes shape; and eversion, when contrac- velopment, but their small size and location inside the larva and tion of the PE is thought to drive the appendages through the pupa has prevented live imaging techniques from extensively be- larval epidermis (12) (reviewed in refs. 13, 14). The interaction ing used in their study. Here, we introduce the use of viscous between the PE and the larval epidermis has been described to culture medium to enable high resolution imaging of imaginal disc be essential (15), as is the partial invasion of the larval walls by development. As a proof of principle, we have analyzed the trans- the stalk cells (15), but the difficulty of analyzing this dynamic formation that occurs during metamorphosis of the wing imaginal process in fixed tissue has prevented a full analysis of the cellular disc into the mature wing and report several previously unob- changes that occur. -

The Drosophila JNK Pathway Controls the Morphogenesis of Imaginal Discs During Metamorphosis
Development 126, 5453-5462 (1999) 5453 Printed in Great Britain © The Company of Biologists Limited 1999 DEV5358 The Drosophila JNK pathway controls the morphogenesis of imaginal discs during metamorphosis François Agnès1, Magali Suzanne1 and Stéphane Noselli1,2,* 1Centre de Biologie du Développement, UMR 5547, 118 route de Narbonne, 31062 Toulouse cedex, France 2Harvard Medical School, Department of Genetics, 200 Longwood Avenue, Boston, MA 02115, USA *Author for correspondence (e-mail: [email protected]) Accepted 21 September; published on WWW 9 November 1999 SUMMARY In Drosophila, the Jun-N-terminal Kinase-(JNK) signaling peripodial cells that ultimately delineates the margins of pathway is required for epithelial cell shape changes during fusing discs. In further support of a role of puc in discs dorsal closure of the embryo. In the absence of JNK morphogenesis, pupal and adult hep phenotypes are pathway activity, as in the DJNKK/hemipterous (hep) suppressed by reducing puc function, indicative of a mutant, the dorsolateral ectodermal cells fail both to negative role of puc in disc morphogenesis. Furthermore, elongate and move toward the dorsal midline, leading to we show that the small GTPase Dcdc42, but not Drac1, is dorsally open embryos. We show here that hep and the JNK an activator of puc expression in a hep-dependent manner pathway are required later in development, for correct in imaginal discs. Altogether, these results demonstrate a morphogenesis of other epithelia, the imaginal discs. new role for the JNK pathway in epithelial morphogenesis, During metamorphosis, the imaginal discs undergo and provide genetic evidence for a role of the peripodial profound morphological changes, giving rise to the adult membrane in disc morphogenesis. -

Control of Drosophila Wing Size by Morphogen Range and Hormonal Gating
Control of Drosophila wing size by morphogen range and hormonal gating Joseph Parkera,1,2 and Gary Struhla,b,2 aDepartment of Genetics and Development, Vagelos College of Physicians and Surgeons, Columbia University, New York, NY 10032; and bMortimer B. Zuckerman Mind Brain Behavior Institute, Columbia University, New York, NY 10027 Contributed by Gary Struhl, October 25, 2020 (sent for review August 28, 2020; reviewed by Peter A. Lawrence and Gines Morata) The stereotyped dimensions of animal bodies and their compo- 23) and 2) by inducing growth and vg expression in surrounding nent parts result from tight constraints on growth. Yet, the “pre-wing” cells defined as such by their capacity to initiate Vg mechanisms that stop growth when organs reach the right size expression and enter the wing in response to Dpp and Wg (Fig. are unknown. Growth of the Drosophila wing—a classic paradigm—is 1G, green mode) (19–21). Both mechanisms depend on the ca- governed by two morphogens, Decapentaplegic (Dpp, a BMP) and pacity of vg to autoregulate its own expression via a cis-acting Wingless (Wg, a Wnt). Wing growth during larval life ceases when “quadrant” enhancer (QE; Fig. 1 C and D) (13, 19, 20). In the the primordium attains full size, concomitant with the larval-to- first case, the autoregulatory circuit is intracellular and direct, pupal molt orchestrated by the steroid hormone ecdysone. Here, requiring Vg to act on the QE to maintain vg transcription in we block the molt by genetically dampening ecdysone production, wing cells in response to Dpp and Wg. -

Single-Cell Transcriptomics of the Drosophila Wing Disc Reveals Instructive Epithelium-To-Myoblast Interactions
RESEARCH ARTICLE Single-cell transcriptomics of the Drosophila wing disc reveals instructive epithelium-to-myoblast interactions Nicholas J Everetts1,2†, Melanie I Worley1†, Riku Yasutomi1, Nir Yosef2*, Iswar K Hariharan1* 1Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, United States; 2Department of Electrical Engineering & Computer Science, Center for Computational Biology, UC Berkeley, University of California, Berkeley, Berkeley, United States Abstract In both vertebrates and invertebrates, generating a functional appendage requires interactions between ectoderm-derived epithelia and mesoderm-derived cells. To investigate such interactions, we used single-cell transcriptomics to generate a temporal cell atlas of the Drosophila wing disc from two developmental time points. Using these data, we visualized gene expression using a multilayered model of the wing disc and cataloged ligand–receptor pairs that could mediate signaling between epithelial cells and adult muscle precursors (AMPs). We found that localized expression of the fibroblast growth factor ligands, Thisbe and Pyramus, in the disc epithelium regulates the number and location of the AMPs. In addition, Hedgehog ligand from the epithelium activates a specific transcriptional program within adjacent AMP cells, defined by AMP- specific targets Neurotactin and midline, that is critical for proper formation of direct flight *For correspondence: [email protected] (NY); muscles. More generally, our annotated temporal cell atlas provides an organ-wide view of [email protected] (IKH) potential cell–cell interactions between epithelial and myogenic cells. †These authors contributed equally to this work Competing interests: The Introduction authors declare that no The development of multicellular eukaryotes gives rise to organs that are composed of cells of many competing interests exist. -
Wing Disks.Pdf
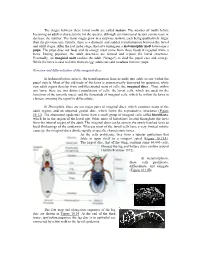
The stages between these larval molts are called instars. The number of molts before becoming an adult is characteristic for the species, although environmental factors can increase or decrease the number. The instar stages grow in a stepwise fashion, each being qualitatively larger than the previous one. Finally, there is a dramatic and sudden transformation between the larval and adult stages. After the last instar stage, the larva undergoes a metamorphic molt to become a pupa. The pupa does not feed, and its energy must come from those foods it ingested while a larva. During pupation, the adult structures are formed and replace the larval structures. Eventually, an imaginal molt enables the adult ("imago") to shed the pupal case and emerge. While the larva is said to hatch from an egg, adults are said to eclose from the pupa. Eversion and differentiation of the imaginal discs In holometabolous insects, the transformation from juvenile into adult occurs within the pupal cuticle. Most of the old body of the larva is systematically destroyed by apoptosis, while new adult organs develop from undifferentiated nests of cells, the imaginal discs . Thus, within any larva, there are two distinct populations of cells: the larval cells, which are used for the functions of the juvenile insect, and the thousands of imaginal cells, which lie within the larva in clusters, awaiting the signal to differentiate. In Drosophila, there are ten major pairs of imaginal discs, which construct many of the adult organs, and an unpaired genital disc, which forms the reproductive structures (Figure 18.12). The abdominal epidermis forms from a small group of imaginal cells called histoblasts, which lie in the region of the larval gut. -

Imaginal Pioneers Prefigure the Formation of Adult Thoracic Muscles in Drosophila Melanogaster
Developmental Biology 222, 450–459 (2000) View metadata,doi:10.1006/dbio.2000.9676, citation and similar papers available at onlinecore.ac.uk at http://www.idealibrary.com on brought to you by CORE provided by Elsevier - Publisher Connector Imaginal Pioneers Prefigure the Formation of Adult Thoracic Muscles in Drosophila melanogaster Patricia K. Rivlin, Anne M. Schneiderman, and Ronald Booker Section of Neurobiology and Behavior, Cornell University, W121 Seeley G. Mudd Hall, Ithaca, New York 14853-2702 In insects, specialized mesodermal cells serve as templates to organize myoblasts into distinct muscle fibers during embryogenesis. In the grasshopper embryo, large mesodermal cells called muscle pioneers extend between the epidermal attachment points of future muscle fibers and serve as foci for myoblast fusion. In the Drosophila embryo, muscle founder cells serve a similar function, organizing large numbers of myoblasts into larval muscles. During the metamorphosis of Drosophila, nearly all larval muscles degenerate and are replaced by a set of de novo adult muscles. The extent to which specialized mesodermal cells homologous to the founders and pioneers of the insect embryo are involved in the development of adult-specific muscles has yet to be established. In the larval thorax, the majority of imaginal myoblasts are associated with the imaginal discs. We report here the identification of a morphologically distinct class of disc-associated myoblasts, which we call imaginal pioneers, that prefigures the formation of at least three adult-specific muscles, the tergal depressor of the trochanter and dorsoventral muscles I and II. Like the muscle pioneers of the grasshopper, the imaginal pioneers attach to the epidermis at sites where the future muscle insertions will arise and erect a scaffold for developing adult muscles. -

Perspectives
PERSPECTIVES overabundance of individuals within a species; OPINION differential survival of those most fit for the environmental circumstances; and the inheri- tance of those traits by the progeny of the sur- Opening Darwin’s black box: vivours. The molecular version of the modern synthesis showed that evolution can even teaching evolution through work over small timescales and therefore clearly showed how the principles of genetics developmental genetics and natural selection can, for example, explain the origins of pesticide-resistant insects and antibiotic-resistant bacteria2–4. Scott F. Gilbert The existence and efficacy of microevolu- tion is widely accepted, even by most cre- When biologists are asked to discuss the evolution. I discuss one possible way — there ationists5.But creationists are not concerned evidence for evolution at public forums, they are certainly others. about antibiotic-sensitive bacteria becoming usually use well-established microevolutionary Evolution has generated our planet’s biodi- antibiotic-resistant or about the beak shape examples. Although these examples show versity, and over the past century scientists changes of Galapagos finch species. They the efficacy of evolution within species, they have become able to explain the mechanisms have remained bacteria and finches, respec- often leave audiences susceptable to the by which changes in animal body structure can tively, so nothing much has changed — no arguments of creationists who deny that be produced, inherited and selected. Genetics new species has been created. To creationists, evolution can create new structures and is crucial to this understanding. The MODERN the synthesis of evolution and genetics can- species. Recent studies from evolutionary SYNTHESIS initially explained evolution through not explain how some fish became amphib- developmental biology are beginning to the mathematics of population genetics. -

The Roles of the Homeobox Genes Aristaless and Distal-Less In
Development 125, 4483-4493 (1998) 4483 Printed in Great Britain ©The Company of Biologists Limited 1998 DEV7610 The roles of the homeobox genes aristalessand Distal-less in patterning the legs and wings of Drosophila Gerard Campbell*,‡ and Andrew Tomlinson Department of Genetics and Development, Center for Neurobiology and Behavior, Columbia University College of Physicians and Surgeons, HHSC, Room 1120, New York, NY 10032, USA *Address from 1st December 1998: Department of Biological Sciences, University of Pittsburgh, Pittsburgh, PA 15260, USA ‡Author for correspondence (e-mail: [email protected]) Accepted 1 September; published on WWW 20 October 1998 SUMMARY In the leg and wing imaginal discs of Drosophila, the are the only regions in which Dllfunction is required. expression domains of the homeobox genes aristaless(al) However, earlier in development Dllis required in more and Distal-less(Dll) are defined by the secreted signaling proximal regions of the leg suggesting it is expressed at high molecules Wingless (Wg) and Decapentaplegic (Dpp). levels in these cells early in development but not later. This Here, the roles played by aland Dllin patterning the legs reveals a correlation between a temporal requirement for and wings have been investigated through loss of function Dlland position along the proximodistal axis; how this may studies. In the developing leg, alis expressed at the relate to the generation of the P/D axis is discussed. Dllis presumptive tip and a molecularly defined null allele of al required in the distal regions of the leg for the expression reveals that its only function in patterning the leg appears of tarsal-specific genes including aland bric-a-brac. -

Pattern Regulation in Imaginal Discs 1593
Development 126, 1591-1599 (1999) 1591 Printed in Great Britain © The Company of Biologists Limited 1999 DEV5294 Hedgehog is required for activation of engrailed during regeneration of fragmented Drosophila imaginal discs Matthew C. Gibson* and Gerold Schubiger Department of Zoology, Box 351800, University of Washington, Seattle, WA 98195, USA *Author for correspondence (e-mail: [email protected]) Accepted 4 February; published on WWW 17 March 1999 SUMMARY Surgically fragmented Drosophila appendage primordia activity, prothoracic leg disc fragments fail to undergo (imaginal discs) engage in wound healing and pattern anterior/posterior conversion, but can still regenerate regulation during short periods of in vivo culture. missing anterior pattern elements. We suggest that Prothoracic leg disc fragments possess exceptional hedgehog-independent regeneration within the anterior regulative capacity, highlighted by the ability of anterior compartment (termed integration) is mediated by the cells to convert to posterior identity and establish a novel positional cues encoded by wingless and decapentaplegic. posterior compartment. This anterior/posterior conversion Taken together, our results provide a novel mechanistic violates developmental lineage restrictions essential for interpretation of imaginal disc pattern regulation and normal growth and patterning of the disc, and thus permit speculation that similar mechanisms could govern provides an ideal model for understanding how cells change appendage regeneration in other organisms. -

Control of Drosophila Adult Pattern by Extradenticle
Development 121, 2117-2125 (1995) 2117 Printed in Great Britain © The Company of Biologists Limited 1995 Control of Drosophila adult pattern by extradenticle Sergio González-Crespo and Ginés Morata Centro de Biología Molecular, Consejo Superior de Investigaciones Científicas, Universidad Autónoma de Madrid, 28049 - Madrid, Spain SUMMARY The homeobox gene extradenticle (exd) acts as a cofactor of required for functions normally not associated with the homeotic genes in the specification of larval patterns homeosis, such as the maintenance of the dorsoventral during embryogenesis. To study its role in adult patterns, pattern, the specification of subpatterns in adult we have generated clones of mutant exd− cells and appendages or the arrangement of bristles in the examined their effect on the different body parts. In some mesonotum and genitalia. Our findings indicate that exd is regions, exd− clones exhibit homeotic transformations critically involved in adult morphogenesis, not only in the similar to those produced by known homeotic mutations homeotic function but also in several other developmental such as Ultrabithorax (Ubx), labial (lab), spineless-arista- processes. pedia (ssa) or Antennapedia (Antp). In other regions, the lack of exd causes novel homeotic transformations Key words: cofactor, clonal analysis, homeotic genes, imaginal producing ectopic eyes and legs. Moreover, exd is also discs, human pbx genes, Drosophila, extradenticle INTRODUCTION similar DNA-binding specificities in vitro (Hoey and Levine, 1988) again posing the question as to how the morphological A small number of primary homeotic genes in the Antenna- diversity is encoded. pedia-Complex (ANT-C) and the Bithorax-Complex (BX-C) One factor contributing to the specificity of homeotic gene specify the identity of segments of Drosophila (Lewis, 1978; function appears to be the product of the gene extradenticle Sánchez-Herrero et al., 1985; Kaufman et al., 1990). -

Divergent Mechanisms for Regulating Growth and Development After Imaginal Disc Damage in the Tobacco Hornworm, Manduca Sexta Manuel A
© 2019. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2019) 222, jeb200352. doi:10.1242/jeb.200352 RESEARCH ARTICLE Divergent mechanisms for regulating growth and development after imaginal disc damage in the tobacco hornworm, Manduca sexta Manuel A. Rosero1, Benedict Abdon1, Nicholas J. Silva1, Brenda Cisneros Larios1, Jhony A. Zavaleta1, Tigran Makunts1, Ernest S. Chang2, S. Janna Bashar1, Louie S. Ramos1, Christopher A. Moffatt1 and Megumi Fuse1,* ABSTRACT ablation experiments have demonstrated the importance of these Holometabolous insects have been able to radiate to vast ecological discs to the coupling of growth and metamorphosis when injury niches as adults through the evolution of adult-specific structures such results in a developmental delay. These delays presumably provide as wings, antennae and eyes. These structures arise from imaginal sufficient time for tissue regeneration. Injury to imaginal discs discs that show regenerative capacity when damaged. During imaginal has also been shown to delay development in a variety of disc regeneration, development has been shown to be delayed in the holometabolous insects, whether through genetic tissue ablation fruit fly Drosophila melanogaster, but how conserved the delay- (Colombani et al., 2012; Hackney et al., 2012), surgical ablation or ’ inducing mechanisms are across holometabolous insects has not autotomy (Kunkel, 1977; O Farrell and Stock, 1953, 1954; Stock ’ been assessed. The goal of this research was to develop the hornworm and O Farrell, 1954), or X-ray-induced selective damage (Ely and ’ Manduca sexta as an alternative model organism to study such Jungreis, 1977; O Brien and Wolf, 1964; Poodry and Woods, 1990; damage-induced mechanisms, with the advantage of a larger Stieper et al., 2008). -

The YPWM Motif Links Antennapedia to the Basal Transcriptional Machinery
RESEARCH ARTICLE 1669 Development 135, 1669-1679 (2008) doi:10.1242/dev.018028 The YPWM motif links Antennapedia to the basal transcriptional machinery Frédéric Prince1, Tomonori Katsuyama2, Yoshiteru Oshima2, Serge Plaza3, Diana Resendez-Perez4, Meera Berry5, Shoichiro Kurata2 and Walter J. Gehring1,* HOX genes specify segment identity along the anteroposterior axis of the embryo. They code for transcription factors harbouring the highly conserved homeodomain and a YPWM motif, situated amino terminally to it. Despite their highly diverse functions in vivo, HOX proteins display similar biochemical properties in vitro, raising the question of how this specificity is achieved. In our study, we investigated the importance of the Antennapedia (Antp) YPWM motif for homeotic transformations in adult Drosophila. By ectopic overexpression, the head structures of the fly can be transformed into structures of the second thoracic segment, such as antenna into second leg, head capsule into thorax (notum) and eye into wing. We found that the YPWM motif is absolutely required for the eye-to-wing transformation. Using the yeast two-hybrid system, we were able to identify a novel ANTP-interacting protein, Bric-à-brac interacting protein 2 (BIP2), that specifically interacts with the YPWM motif of ANTP in vitro, as well as in vivo, transforming eye to wing tissue. BIP2 is a TATA-binding protein associated factor (also known as dTAFII3) that links ANTP to the basal transcriptional machinery. KEY WORDS: ANTP, HOX, bip2, dTAF3, Drosophila, Eye-to-wing transformation, Homeotic transformation INTRODUCTION in conferring specificity (Lin and McGinnis, 1992; Chan and Mann, Homeotic genes (HOX) are selector genes that generate 1993; Zeng et al., 1993; Chauvet et al., 2000; Gebelein et al., 2002; morphological diversity along the anteroposterior body axis during Merabet et al., 2003).