Euronext Brussels Awards 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UCB SA (Incorporated with Limited Liability in Belgium) As Issuer
Base Prospectus dated 8 March 2021 UCB SA (incorporated with limited liability in Belgium) as Issuer EUR 5,000,000,000 Euro Medium Term Note Programme Due from one month from the date of original issue This base prospectus (the “Base Prospectus”) relating to the EUR 5,000,000,000 Euro Medium Term Note Programme (the “Programme”) of UCB SA, a limited liability company (société anonyme) incorporated under the laws of Belgium, having its registered office at Allée de la Recherche 60, B-1070 Brussels and registered with the Crossroads Bank for Enterprises under number 0403.053.608 (“UCB”, or the “Issuer”) is valid, for the purpose of the admission to trading and listing of the Notes (as defined below) on the regulated market of Euronext Brussels or on another regulated market in the European Economic Area (the “EEA”), for a period of twelve months from the date of approval. Any Notes issued under the Programme are issued subject to the provisions set out herein. Under the Programme, the Issuer, subject to compliance with all relevant laws, regulations and directives, may from time to time issue Euro Medium Term Notes (the “Notes”) as may be agreed between the Issuer and the relevant Dealer (as defined below). The minimum Specified Denomination of Notes shall be EUR 100,000 (or its equivalent in other currencies).The maximum aggregate nominal amount of Notes outstanding will not at any time exceed EUR 5,000,000,000 (or the equivalent in other currencies). This Base Prospectus has been approved as a base prospectus for the purposes of Article 8 of Regulation 2017/1129 (as amended, the “Prospectus Regulation”) on 8 March 2021 by the Belgian Financial Services and Markets Authority (the “FSMA”) in its capacity as competent authority in accordance with Article 20 of the Prospectus Regulation. -

Integrated Annual Report 2020
Amber, living with psoriasis INTEGRATED ANNUAL REPORT 2020 ADAPTING FOR BETTER CARE INTEGRATED ANNUAL REPORT 2020 ADAPTING FOR BETTER CARE Welcome to our Integrated Annual Report 2020! Our Integrated Annual Report 2020 – Adapting for Better Care – aims to provide all interested stakeholders with the best pos- sible information on how UCB is creating value for patients with severe diseases and about how we care for our employees, for communities, and for our planet, now and into the future. About this report This Integrated Annual Report 2020 includes the management report in accordance with article 12 of the Royal Decree of 14 November 2007 relating to the obligations of issuers of finan- cial instruments admitted to trading on a regulated market. All information required to be included in such management report pursuant to articles 3:6 and 3:32 of the Belgian Code of Com- panies and Associations (i.e. Corporate Governance Statement – Remuneration Report included -, Business Performance Review and UCB’s Statement on extra-financial1 information) is reported throughout all different sections of this Integrated Annual Re- port. This Integrated Annual Report together with the materiality assessment have been prepared in accordance with the Global Reporting Standards core option and extra-financial information is audited by a third party. 1 ‘Extra-financial’ is the term used by UCB for information commonly referred to as ‘non-financial’. UCB | Integrated Annual Report 2020 3 Index Key Figures 6 UCB At a Glance 8 Letter to our stakeholders -

Investor Presentation Transforming to a Specialist Wealth Manager
Investor Presentation Transforming to a specialist wealth manager March 2016 Van Lanschot at a glance Van Lanschot’s profile Solid performance on all key financials • One strategy: pure-play, independent wealth manager focusing on 2015 2014 preservation and creation of wealth for our clients • Net profit € 42.8m € 108.7m • Underlying result € 60.1m € 54.2m • Two leading brands: Van Lanschot and Kempen & Co • CET I ratio 16.3% 14.6% • CET I ratio, fully loaded 15.4% 13.4% • Total Capital ratio 17.0% 15.2% • Three core activities: Private Banking, Asset Management and • Leverage ratio, fully loaded 6.1% 5.3% Merchant Banking • Funding ratio 94.1% 95.3% • Client assets € 62.6bn € 58.5bn Good progress with strategy Financial targets 2017 • Private Banking: € 0.3 billion net inflow, € 1.5 billion entrusted to Evi Target van Lanschot, commission income +12% 2015 2017 • Asset Management: acquisition of fiduciary management KCM UK as • Common Equity Tier I ratio 16.3% >15% step stone for further international growth, new mandates won early 2016 adding approximately € 2 billion of AuM • Return on Common Equity Tier I 4.9% 10-12% • Merchant Banking: commission income +28%, solid market share in • Efficiency ratio 74.4% 60-65% selected niches, research coverage expanded • Corporate Banking: initial run-off target achieved, run-off continues Van Lanschot Investor Presentation - March 2016 1 1. Profile & Strategy of Van Lanschot 2. Financial Performance FY2015 Agenda Van Lanschot Investor Presentation - March 2016 2 Transforming from small “universal” -

Download Annual Report 2019
1 ANNUAL REPORT 2019 This document is a translation of the Dutch original and is provided as a courtesy only. In the event of any disparity, the Dutch version shall prevail. No rights may be derived from the translated document. TABLE OF CONTENTS Key figures 6 About BinckBank 7 Report of the Executive Board 8 Notes to the consolidated result for 2019 12 Corporate social responsibility 14 Risk management 18 Corporate governance 24 Message from the chairman of the Supervisory Board 27 Report of the Supervisory Board 28 Personal detail of the boards 30 Consolidated financial statements 32 Consolidated statement of financial position 33 Consolidated income statement 34 Consolidated statement of cash flows 35 Consolidated statement of changes in equity 37 Notes to the consolidated financial statements 38 Notes to the consolidated statement of financial position 50 Notes to the consolidated income statement 63 Other notes to the consolidated financial statements 67 Company financial statements 103 Company balance sheet 104 Company income statement 105 Notes to the company financial statements 106 Notes to the company balance sheet 107 Notes to the company income statement 110 Other information 114 Independent auditor’s report 114 Provisions of the articles of association regarding profit appropriation (Article 27) 120 5 KEY FIGURES for the period ending 31 December, consolidated (amounts in € 000's) 2019 2018 2017 2016 2015 CUSTOMER FIGURES Number of transactions * 10,390,893 9,870,170 7,705,024 7,726,110 9,293,591 Assets under administration -

Powerpoint Presentatie
Analyst Meeting Amsterdam, 1 December 2010 Programme 2.30 pm Ieko Sevinga Private Banking 3.00 pm Arjan Huisman Operations 3.30 pm – 4.00 pm Break 4.00 pm Constant Korthout Funding and Basel III 4.30 pm Floris Deckers Strategy & Outlook for the sector 5.30 pm Drinks & dinner 1 Analyst Meeting Ieko Sevinga, member of the Board of Managing Directors Van Lanschot’s strategy To offer high-quality financial services to wealthy individuals, Mission director-owners and other select client groups Van Lanschot aims to be the best private bank in the Netherlands Vision and Belgium To be able to measure the achievement of its vision, Van Lanschot Targets has formulated targets relating to clients and employees and 2010-2013 financial targets 1. Focus on private banking 2. Enhance commercial effectiveness Strategy 3. Invest continually in service quality 4. Maintain a solid profile Independent Professional Core values Committed Ambitious 3 Strategic priorities Focus on Full-service offering Private Acquisition focused on high net-worth individuals and Banking entrepreneurs and their businesses Enhance Growth of client satisfaction commercial Growth in number of clients effectiveness Growth in revenues Invest Customer care continually in Transparent and good product and service offering service quality Operational excellence Risk management Maintain Cost control a sound Stricter deployment of capital for clients with a view to expected profile higher capital requirements 4 Focused business model FULL SERVICE OFFERING Business Private Banking -

Invitation and Agenda to the Annual General Meeting of Shareholders Of
invitation and agenda to the annual general meeting of shareholders of van lanschot nv, to be held in the auditorium of the van lanschot tower, leonardo da vinciplein 60, ’s-hertogenbosch, the netherlands, on thursday 15 may 2014 at 2 p.m. 1 invitation and agenda ANNUAL GENERAL MEETING OF 9. Extension of powers of the Board of Managing SHAREHOLDERS OF VAN LANSCHOT NV Directors a) Extension of the power of the Board of Managing Directors to issue ordinary shares (voting item) Van Lanschot NV invites its shareholders and holders of b) Extension of the power of the Board of Managing Directors depositary receipts to attend the Annual General to limit or exclude the pre-emption right in the event of the Meeting, to be held in the auditorium of the Van Lanschot issue of ordinary shares (voting item) Tower, Leonardo Da Vinciplein 60, ’s-Hertogenbosch, the Netherlands, on Thursday 15 May 2014 at 2 p.m. 10. Any other business and closure of meeting Availability of meeting documents 1. Opening Prior to the meeting, the following documents can be obtained by the shareholders and holders of depositary receipts at no cost 2. 2013 annual report from the office of Van Lanschot NV (Leonardo da Vinciplein 60, a) Report of the Supervisory Board (discussion item) 5223 DR, ’s-Hertogenbosch, the Netherlands): b) Report of the Board of Managing Directors for 2013 – the agenda with explanatory notes; (discussion item) – the report of the Supervisory Board; c) Strategy implementation status report (discussion item) – the Report of the Board of Managing Directors for 2013; – the 2013 financial statements and other information; 3. -

Euro Stoxx® Multi Premia Index
EURO STOXX® MULTI PREMIA INDEX Components1 Company Supersector Country Weight (%) SARTORIUS STEDIM BIOTECH Health Care France 1.59 IMCD Chemicals Netherlands 1.25 VOPAK Industrial Goods & Services Netherlands 1.15 BIOMERIEUX Health Care France 1.04 REMY COINTREAU Food, Beverage & Tobacco France 1.03 EURONEXT Financial Services France 1.00 HERMES INTERNATIONAL Consumer Products & Services France 0.94 SUEZ ENVIRONNEMENT Utilities France 0.94 BRENNTAG Chemicals Germany 0.93 ENAGAS Energy Spain 0.90 ILIAD Telecommunications France 0.89 DEUTSCHE POST Industrial Goods & Services Germany 0.88 FUCHS PETROLUB PREF Chemicals Germany 0.88 SEB Consumer Products & Services France 0.87 SIGNIFY Construction & Materials Netherlands 0.86 CARL ZEISS MEDITEC Health Care Germany 0.80 SOFINA Financial Services Belgium 0.80 EUROFINS SCIENTIFIC Health Care France 0.80 RATIONAL Industrial Goods & Services Germany 0.80 AALBERTS Industrial Goods & Services Netherlands 0.74 KINGSPAN GRP Construction & Materials Ireland 0.73 GERRESHEIMER Health Care Germany 0.72 GLANBIA Food, Beverage & Tobacco Ireland 0.71 PUBLICIS GRP Media France 0.70 UNITED INTERNET Technology Germany 0.70 L'OREAL Consumer Products & Services France 0.70 KPN Telecommunications Netherlands 0.68 SARTORIUS PREF. Health Care Germany 0.68 BMW Automobiles & Parts Germany 0.68 VISCOFAN Food, Beverage & Tobacco Spain 0.67 SAINT GOBAIN Construction & Materials France 0.67 CORBION Food, Beverage & Tobacco Netherlands 0.66 DAIMLER Automobiles & Parts Germany 0.66 PROSIEBENSAT.1 MEDIA Media Germany 0.65 -

Dynavax Announces Full Enrollment of Phase II/III AIC Trial
Dynavax Announces Full Enrollment of Phase II/III AIC Trial BERKELEY, Calif., May 19 /PRNewswire-FirstCall/ -- Dynavax Technologies Corporation (Nasdaq: DVAX), a biopharmaceutical company focused on the discovery, development, and commercialization of innovative products to treat and prevent allergies, infectious diseases and chronic inflammatory diseases, today announced that its Phase II/III clinical trial of AIC for ragweed allergy, which started in late February 2004, has been fully enrolled. The double blind, placebo controlled study involves 462 subjects at 30 sites in the U.S. and will be completed over the next 18 months. The immunization schedule, consisting of six injections over six weeks, will be completed by June. The clinical trial is being conducted in collaboration with UCB Pharma, a European pharmaceutical company that markets XYZAL® and developed ZYRTEC®, the leading prescription anti- allergy drug worldwide. Dynavax and UCB signed a global licensing and commercialization agreement covering ragweed and grass allergy immunotherapies in February 2004. The primary endpoint of the Phase II/III ragweed study will be nasal symptoms scores after the second ragweed season in the late summer and early fall of 2005, with secondary endpoints assessing symptoms, medication use and quality of life in both the first and second year. A blinded interim analysis will be made in late 2004 to assess safety and the appropriateness of commencing a one-year Phase III trial in early 2005. "The collaboration with UCB has been very effective, with both organizations working diligently to advance our ragweed program," said Dr. Dino Dina, president and CEO of Dynavax. "The rapid enrollment of our ragweed trial, particularly given its size and stringent enrollment criteria, is an early indication of the level of interest that both investigators and patients have in this therapy. -

Achmea Bank N.V. Reports a Positive Result of Eur 37 Million Limited Impact Covid-19 Crisis on Achmea Bank’S Financial Position
ACHMEA BANK N.V. PRESS RELEASE 2020 ACHMEA BANK N.V. REPORTS A POSITIVE RESULT OF EUR 37 MILLION LIMITED IMPACT COVID-19 CRISIS ON ACHMEA BANK’S FINANCIAL POSITION Tilburg, 15 March 2021 • Achmea Bank N.V. reported for 2020 an operating profit of EUR 37 million, EUR 28 million after tax (2019: EUR 50 million, after tax EUR 37 million) • The Common Equity Tier 1 Capital Ratio remains strong at 20.4% (2019: 19.2%) • Achmea Bank further executed its ambition to grow in mortgages, through the acquisition of a portfolio of Dutch residential mortgages from BinckBank of EUR 0.5 billion • Achmea Bank combined its mortgage activities with Syntrus Achmea Real Estate & Finance to focus on growth in mortgages • The Bank positions itself to become a data driven connected bank optimizing its asset base to support the Achmea strategy Achmea Bank reported a profit before tax of EUR 37 million in 2020 (2019 EUR 50 million). The 2019 result included an one-off accounting result of EUR 18 million related to the a.s.r. transaction. The operating result for 2020, excluding one-off results and fair value result, increased from EUR 34 million in 2019 to EUR 42 million in 2020. The increase in operating result is mainly due to a higher interest margin of EUR 16 million. Impairment charges amounted EUR 3 million (2019 EUR +4 million). 2020 was dominated by the Covid-19 crisis. This crisis affects the social and economic living environment and thereby also our customers. Since March 2020, Achmea Bank offered the possibility of a payment holiday to mortgage customers with payment difficulties directly related to the Covid-19 crisis. -

MJ Bijlsma CM Van Den Broek JFG Bruggert E
The following staff of NMa contributed to the realisation of this document: M.J. Bijlsma C.M. van den Broek J.F.G. Bruggert E.J.R. Droste M. Gerritsen W. Meester I.S. Nobel M.M. Oijevaar C. Wolfsen C.J. Zonderland The contents of this publication closed on 1 October 2005. Developments after this date could therefore no longer be included in the texts. 1 Contents Foreword 4 1 The Financial Sector Monitor in 2005 6 1.1 Introduction 6 1.2 Activities of FSM in 2005 6 1.3 Success factors 8 1.4 Structure 9 1.5 Market developments in 2005 10 2 Competition between insurance brokers 15 2.1 Introduction 15 2.2 Responses to the consultation document 15 2.3 Survey 16 2.4 Analysis of consumer choice 20 2.5 Conclusions 24 3 Effects of the transfer of PIN contracts 26 3.1 Introduction 26 3.2 Outcomes of the research by NIPO/ECORYS-NEI 27 3.3 Outcomes of FSM's research into tariffs 29 3.4 Conclusions 30 4 Entry and exit of banks 32 4.1 Introduction 32 4.2 Registrations and deregistration under the Credit System (Supervision) Act 32 4.2.1 Breakdown according to the type of institution 34 4.2.2 Registrations and Deregistrations of Dutch commercial banks according to their background 36 4.3 Entries and exits 37 4.4 Conclusions 38 5 The geographical dimension of the health insurance market 39 5.1 Introduction 39 5.2 The healthcare market 40 5.3 Relevant geographical dimension 42 5.4 Dynamic factors 44 5.4.1 Regional mechanism 44 5.4.2 Counteracting factors 47 5.4.3 Empirical research 49 5.5 Conclusions 52 6 Interbank charges: economic theory and international -

Purpose & Patience
PURPOSE & PATIENCE We aspire to be the preferred partner of entrepreneurs and families who lead growing companies by backing them with patient capital and supportive advice — Q1 2020 Edition — OUR MISSION Our goal at Sofina is to create economic value with a human approach We believe that the entrepreneurial spirit that characterises many family businesses and growth companies is a source of progress. By supporting these entrepreneurs and innovators, we intend to contribute to global growth, development and innovation. We believe entrepreneurs become successful by being competitive in a globalised market Our mission is to provide patient capital, expertise and advice to growing companies led by entrepreneurs and families. We aspire to be their preferred partner, and have a long-term horizon that few other investors can match. Our heritage and culture are what make us unique We put human relationships at the heart of what we do. All our investments are stories of shared values, friendships and ambitious projects with talented entrepreneurs and their management teams. By continuously working in this way, we aspire to become the preferred investment partner of those sharing our beliefs and vision. 2 SOFINA - PURPOSE & PATIENCE OUR MISSION 3 KEY FIGURES Highlights EUR 7.63 BN GLOBAL SHAREHOLDERS’ EQUITY REACH A FAMILY RUN 3 US EUROPE ASIA AND CONTROLLED COMPLEMENTARY Change over the last 20 INVESTMENT COMPANY INVESTMENT years (2) STYLES 8.00 7.00 6.00 5.00 FOUR FOCUS 4.00 SECTORS Long-term minority 3.00 investments 2.00 1.00 43% OF SHAREHOLDERS’ EQUITY (1) Offices in Brussels, 0 Luxembourg and Singapore Consumer and Retail 1999 2019 Sofina Private Funds – Investments in venture and growth capital funds Digital Transformation 31% OF SHAREHOLDERS’ EQUITY (1) Roots going back 26 Sofina Growth – Investments in Education investment fast-growing businesses professionals 120+ YEARS across our 20% OF SHAREHOLDERS’ EQUITY (1) 3 offices Healthcare (1) Considering the portfolio in transparency. -
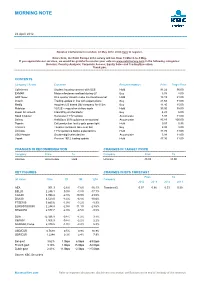
Analyst Report KBC Securities
MORNING NOTE 24 April 2012 Benelux Conference in London, 22 May 2012. Click here to register. Dear client, the Extel Europe 2012 survey will run from 19 March to 4 May. If you appreciate our services, we would be grateful to receive your vote on www.extelsurvey.com in the following categories: Benelux: Country Analysis; Corporate Access; Equity Sales and Trading/Execution. Thank you. CONTENTS Company / Sector Comment Recommendation Price Target Price Cofinimmo Student housing contract with ULB Hold 91.22 96.00 EXMAR Major milestones realised during Q1 Buy 5.76 8.00 GDF Suez One quarter doesn't make the trend reversal Hold 18.19 21.00 Imtech Trading update in line with expectations Buy 21.65 31.00 Mediq Acquires US based D&I company for €12m Buy 11.40 15.00 Mobistar 1Q12E – regulation strikes again Hold 33.00 36.00 Quest for Growth Clear2Pay on the block? Buy 4.23 6.00 Reed Elsevier Reiterates FY12 outlook Accumulate 8.98 11.00 Solvay Ambitious 2016 guidance announced Accumulate 85.83 100.00 Tigenix Cell production facility gets green light Hold 0.57 0.95 Transics Tavares considers take-over bid Buy 6.95 8.00 Umicore FY12 guidance below expectations Hold 41.76 43.00 USG People Double-digit sales decline Accumulate 5.98 11.00 Vopak Preview 1Q12 trading update Hold 47.30 41.00 CHANGES IN RECOMMENDATION CHANGES IN TARGET PRICE Company From To Company From To Umicore Accumulate Hold Umicore 45.00 43.00 KEY FIGURES CHANGES IN EPS FORECAST From To (at close) Price 1D 1M 12M Company 2012 2013 2012 2013 AEX 301.3 -2.6% -7.6% -16.1% Transics (€)