Eruption of Bioengineered Teeth: a New Approach Based on a Polycaprolactone Biomembrane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Eruption Abnormalities in Permanent Molars: Differential Diagnosis and Radiographic Exploration
DOI: 10.1051/odfen/2014054 J Dentofacial Anom Orthod 2015;18:403 © The authors Eruption abnormalities in permanent molars: differential diagnosis and radiographic exploration J. Cohen-Lévy1, N. Cohen2 1 Dental surgeon, DFO specialist 2 Dental surgeon ABSTRACT Abnormalities of permanent molar eruption are relatively rare, and particularly difficult to deal with,. Diagnosis is founded mainly on radiographs, the systematic analysis of which is detailed here. Necessary terms such as non-eruption, impaction, embedding, primary failure of eruption and ankylosis are defined and situated in their clinical context, illustrated by typical cases. KEY WORDS Molars, impaction, primary failure of eruption (PFE), dilaceration, ankylosis INTRODUCTION Dental eruption is a complex developmen- at 0.08% for second maxillary molars and tal process during which the dental germ 0.01% for first mandibular molars. More re- moves in a coordinated fashion through cently, considerably higher prevalence rates time and space as it continues the edifica- were reported in retrospective studies based tion of the root; its 3-dimensional pathway on orthodontic consultation records: 2.3% crosses the alveolar bone up to the oral for second molar eruption abnormalities as epithelium to reach its final position in the a whole, comprising 1.5% ectopic eruption, occlusion plane. This local process is regu- 0.2% impaction and 0.6% primary failure of lated by genes expressing in the dental fol- eruption (PFE) (Bondemark and Tsiopa4), and licle, at critical periods following a precise up to 1.36% permanent second molar iim- chronology, bilaterally coordinated with fa- paction according to Cassetta et al.6. cial growth. -

Tooth Eruption and Movement
Tooth eruption and movement Dr. Krisztián Nagy CÍM beírása!!! DÁTUM Diphydont dentition Deciduous dentition – primary dentition CÍM beírása!!! DÁTUM Diphydont dentition Permanent dentition – secondary dentition CÍM beírása!!! DÁTUM Mixed Dentition: Presence of both dentitions CÍM beírása!!! DÁTUM Tooth eruption CÍM beírása!!! DÁTUM • Teeth are formed in relation to the alveolar process. • Epithelial thickening: Dental lamina • Enamel organs: Series of 10 local thickenings on dental lamina in each alveolar process. • Each thickening forms one milk tooth. CÍM beírása!!! DÁTUM Stages in the formation of a tooth germ CÍM beírása!!! DÁTUM Formation of enamel organs CÍM beírása!!! DÁTUM Stages Bud stage : • Characterized by formation of a tooth bud. • The epithelial cells begin to proliferate into the ectomesenchyme of the jaw. CÍM beírása!!! DÁTUM Cap stage : • Formation of dental papilla. • The enamel organ & dental papilla forms the tooth germ. • Formation of ameloblasts. • Formation of odontoblasts. CÍM beírása!!! DÁTUM Bell stage: The cells on the periphery of the enamel organ separate into three important layers: • Cuboidal cells on the periphery of the dental organ form the outer enamel epithelium. • The cells of the enamel organ adjacent to the dental papilla form the inner enamel epithelium. • The cells between the inner enamel epithelium and the stellate reticulum form a layer known as the stratum intermedium. The dental lamina begin to disintegrates, leaving the developing teeth completely separated from the epithelium of the oral cavity. CÍM beírása!!! DÁTUM Crown stage : 1. Mineralization of hard tissues occur. 2. The inner enamel epithelial cells change in shape from cuboidal to columnar. The nuclei of these cells move closer to the stratum intermedium and away from the dental papilla. -

Veterinary Dentistry Basics
Veterinary Dentistry Basics Introduction This program will guide you, step by step, through the most important features of veterinary dentistry in current best practice. This chapter covers the basics of veterinary dentistry and should enable you to: ü Describe the anatomical components of a tooth and relate it to location and function ü Know the main landmarks important in assessment of dental disease ü Understand tooth numbering and formulae in different species. ã 2002 eMedia Unit RVC 1 of 10 Dental Anatomy Crown The crown is normally covered by enamel and meets the root at an important landmark called the cemento-enamel junction (CEJ). The CEJ is anatomically the neck of the tooth and is not normally visible. Root Teeth may have one or more roots. In those teeth with two or more roots the point where they diverge is called the furcation angle. This can be a bifurcation or a trifurcation. At the end of the root is the apex, which can have a single foramen (humans), a multiple canal delta arrangement (cats and dogs) or remain open as in herbivores. In some herbivores the apex closes eventually (horse) whereas whereas in others it remains open throughout life. The apical area is where nerves, blood vessels and lymphatics travel into the pulp. Alveolar Bone The roots are encased in the alveolar processes of the jaws. The process comprises alveolar bone, trabecular bone and compact bone. The densest bone lines the alveolus and is called the cribriform plate. It may be seen radiographically as a white line called the lamina dura. -

Uprighting an Impacted Permanent Mandibular First Molar Associated with a Dentigerous Cyst and a Missing Second Mandibular Molar—A Case Report
dentistry journal Case Report Uprighting an Impacted Permanent Mandibular First Molar Associated with a Dentigerous Cyst and a Missing Second Mandibular Molar—A Case Report Konstantina Tsironi 1,* , Emmanouil Inglezos 1, Emmanouil Vardas 2 and Anastasia Mitsea 3 1 Posidonos 14, Imia square, Voula, 16673 Athens, Greece 2 Clinic of Hospital Dentistry, Dental School, National and Kapodistrian University of Athens, Thivon 2 Goudi, 11527 Athens, Greece 3 Department of Oral Diagnosis and Radiology, Dental School, National and Kapodistrian University of Athens, Thivon 2 Goudi, 11527 Athens, Greece * Correspondence: [email protected]; Tel.: +30-698-682-7064 Received: 3 April 2019; Accepted: 21 May 2019; Published: 27 June 2019 Abstract: The purpose of this paper is to present a case of an impacted mandibular first molar associated with a dentigerous cyst and a missing mandibular second molar in an 11-year-old girl that was treated with combined surgical and orthodontic procedures. After clinical and radiographic evaluation, marsupialization of the cyst was decided, and a molar attachment was bonded on the buccal side of the impacted molar as a part of a full orthodontic treatment with fixed appliances. After 18 months of orthodontic traction, the molar was moved to a more advantageous position, and new bone apposition was observed on the site of the cystic lesion. Histological examination confirmed a dentigerous cyst. The molar was left to erupt spontaneously for 14 more months. A functional occlusion was finally achieved. An interdisciplinary approach proved to be an effective modality in treating a large dentigerous cyst associated with a deeply impacted first mandibular molar, presenting many advantages, such as new bone apposition and patient comfort. -

Tooth Eruption Disorders Associated with Systemic and Genetic Diseases: Clinical Guide
DOI: 10.1051/odfen/2018129 J Dentofacial Anom Orthod 2017;20:402 © The author Tooth eruption disorders associated with systemic and genetic diseases: clinical guide C. Choukroune Qualified specialist in Dentofacial Orthopedics, former Hospital Resident, private practice in Boulogne-Billancourt SUMMARY Tooth eruption is defined as the movement of the dental root and the tooth from its original devel- opment site in the alveolar process to its functional position in the oral cavity. Despite vast amounts of research, the exact mechanism of tooth eruption remains unknown. The authors have shown that the dental crown is not necessary for tooth eruption, whereas the dental follicle seems to be essential for the process. The formation of an eruption pathway by bone resorption allows the root to breach the oral cavity, at the same time, bone formation occurs at the basal level of the dental root. Multiples genetic and molecular structures coordinate these events. Sometimes it is by studying pathological conditions that we discover the essential interactions that occur during tooth eruption. Frequently, a delayed tooth eruption (DTE) is the first, if not the only, expression of a local or general pathology. A DTE can affect directly the diagnosis, the treatment planning, or the timing of the orthodontic treatment. Therefore, it is essential for the orthodontist to identify the cause of a DTE for implementing the correct treatment. KEY WORDS Tooth eruption, genetic disease inborn, systemic disease, delayed tooth eruption INTRODUCTION Dental eruption is a unique physiolog- between osteoblasts, osteoclasts, and the ical event; the tooth is the only organ to dental follicle (DF), involving many genet- appear a few months or years after birth. -

Tooth Eruption
Dr Sameshima CBY 579 lecture notes • Chronology • Biology • Ankylosis • Infraocclusion or submerged teeth • Primary Failure of Eruption • Tooth Migration Classic ADA North American Standards for Tooth Development Eruption sequence • Maxillary teeth: 6 1 2 4 5 3 7 • Mandibular teeth: 6 1 2 3 4 5 7 • Females develop slightly earlier than males Standards on based on data several decades old in the US using Caucasian populations of Northern European ancestry 1 Dr Sameshima CBY 579 lecture notes HAVE THERE BEEN ANY CHANGES REPORTED IN THE LAST FEW DECADES? Emergence of permanent teeth and dental age in a series of Finns – Nystrom et al. Acta Odontologica Scandinavia April 2001. 68% of children – lower 1s erupted before 6s – shift in emergence order in last 30 years New standards for emergence of permanent teeth in Australians – Diamanti and Townsend. Australian Dental J. 2008. Eruption rate of all permanent teeth delayed compared to data from previous years. Expected location of neonatal line The Consideration of Dental Development In Serial extraction - Moorrees CA, Fanning EA, Gron AM. AJO 1963. OLD BUT STILL USEFUL 2 Dr Sameshima CBY 579 lecture notes The Consideration of Dental Development In Serial extraction - Moorrees CA, Fanning EA, Gron AM. AJO 1963. The Consideration of Dental Development In Serial extraction - Moorrees CA, Fanning EA, Gron AM. AJO 1963. The Consideration of Dental Development In Serial extraction - Moorrees CA, Fanning EA, Gron AM. AJO 1963. 3 Dr Sameshima CBY 579 lecture notes The Consideration of Dental Development In Serial extraction - Moorrees CA, Fanning EA, Gron AM. AJO 1963. BIOLOGY OF TOOTH ERUPTION • Definition: movement of a tooth from its site of development within the alveolar process. -
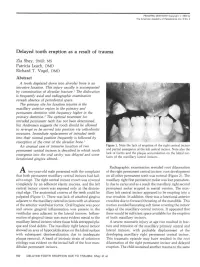
Delayed Tooth Eruption As a Result of Trauma
PEDIATRIC DENTISTRY Copyright & 1983 by The American Academy of Pedodontics Vol. 5 No. 2 Delayed tooth eruption as a result of trauma Zia Shey, DMD, MS Patricia Leach, DMD Richard T. Vogel, DMD Abstract A tooth displaced down into alveolar bone is an intrusive luxation. This injury usually is accompanied by comminution of alveolar fracture.1 The dislocation is frequently axial and radiographic examination reveals absence of periodontal space. The primary site for luxation injuries is the maxillary anterior region in the primary and permanent dentition with frequency higher in the primary dentition.2 The optimal treatment for intruded permanent teeth has not been determined, but Andreasen suggests the tooth should be allowed to re-erupt or be moved into position via orthodontic measures. Immediate replacement of intruded teeth into their normal position frequently is followed by resorption of the crest of the alveolar bone.1 An unusual case of intrusive luxation of two Figure 1. Note the lack of eruption of the right central incisor permanent central incisors is described in which tooth and partial emergence of the left central incisor. Note also the emergence into the oral cavity was delayed and some lack of fornix and the plaque accumulation on the labial sur- faces of the maxillary lateral incisors. keratinized gingiva altered. Radiographic examination revealed root dilaceration ten-year-old male presented with the complaint of the right permanent central incisor; root development that both permanent maxillary central incisors had fail- on all other permanent teeth was normal (Figure 2). The ed to erupt. The right central incisor crown was covered maxillary right first permanent molar was lost premature- completely by an adherent elastic mucosa, and the left ly due to caries and as a result the maxillary right second central incisor crown was exposed only at the distoin- permanent molar erupted in mesial version. -

Signs of Tooth Eruption in Infants
Modern Approaches in Dentistry and L UPINE PUBLISHERS Oral Health Care Open Access DOI: 10.32474/MADOHC.2018.03.000162 ISSN: 2637-4692 Opinion Signs of Tooth Eruption in Infants Karimi* Sepideh Dental Clinic, Department of Pediatric Dentistry, Iran Received: October 16, 2018; Published: October 23, 2018 *Corresponding author: Karimi, Sepideh Dental Clinic, Department of Pediatric Dentistry, Iran Abstract Teething is one of the challenges of medical science in Infancy period. Many studies show that teeth eruptions might have a lot of symptoms, including bad mood, skin rashes on the cheeks and around the mouth, increasing saliva secretion, swollen gums, Sucking anda finger try toand exit etc. from These the signs swollen and gingiva.symptoms are mild in some infants, and in others may be severe; it is difficult for parents to tolerate these conditions. The peak of the severity of teething-related symptoms is when the dental buds have grown sufficiently in the gum Keywords: Teething; Saliva Secretion; Bad Mood; Skin Rash; Swollen Gum; Dental Buds Introduction Signs and Symptoms both parents and children. Note that maintaining the health of teeth Given that the primary teeth are much smaller when they come As important as it is, tooth eruption is a matter of difficulty for and gums in the child is the basis of the health of his teeth and gums out of the gum, and the process of tooth eruption is different in for whole life. Teeth eruption is one of the most important stages children, the symptoms that can usually be expected are: of the growth, and through this stage, the baby will be able to have a) Distracted Dreams: In some children, teething might be a chewable foods. -

Cementum.Pdf
DR JEBIN,MDS.,D.ICOI The periodontium is defined as those tissues supporting and investing the tooth. • It consists of the cementum, • the periodontal ligament, • the alveolar bone and •the gingiva. Cementum is the least understood one of these four tissues. • The Cementum is a hard, avascular, calcified connective tissue covering the root dentin and gives insertion to the periodontal fiber bundle. • It can be regarded as a “bone of attachment”. • It is pale yellow and softer than dentin. • Cementum is formed throughout life and is resistant to resorption. • Cementum functions as an area of attachment for the periodontal ligament fibers. Thickness of cementum: • The thickness of cementum varies considerably coronal third may be 16-60 µm thick (thickness of hair) apical third and furcation areas can be 150-200 µm or even thicker (greatest thickness) • It is thicker in distal surfaces than in mesial surfaces. • Between 11 and 70 years, the average thickness of cementum increases threefold, with greatest increase in apical region. • Average thickness: 95 µm at 20yrs and 215 µm at 60yrs Permanent teeth contains • 45-50% inorganic substance • 50-55% organic material and water. • The inorganic portion consists calcium and phosphate in the form of hydroxyapatite (Ca10[Po4]6[OH]2) • They are thin, needle-shaped crystals. • It is less than that of bone(65%) enamel(97%) dentin(70%) a • The organic portion of the cementum is composed primarily of type I (90%) and type III (about 5%) collagens. • Sharpey’s fibers, which constitute the major portion of cementum are composed of mainly collagen type I. -
Recent Perspectives Vis-À-Vis the Biological Basis of Tooth Eruption
238 > CLINICAL REVIEW Recent perspectives vis-à-vis the biological basis of tooth eruption SADJ July 2015, Vol 70 no 6 p238 - p241 S Nel1, HD Hendrik2, SC Boy3, EJ Raubenheimer4 ABSTRACT A thorough understanding of recent advancements ACRONYMS regarding the molecular interactions responsible for tooth BMP-2: Bone Morphogenetic Protein-2 eruption is indispensable to all dental specialties and may CSF-1: Colony-stimulating Factor-1 provide insight for treating clinical eruption disorders. The biological processes responsible for tooth eruption have DF: Dental Follicle long been a matter of debate. Several types of cells of EGF: Epidermal Growth Factor dental origin and numerous molecular factors that were ERS: Epithelial Root Sheath believed to be responsible for this process have repeatedly Interleukin-1 been considered and investigated. Most existing eruption IL-1α : α theories have concentrated on selective cells or processes MCP-1: Monocyte Chemotactic Protein-1 as the sole generating forces of tooth eruption. This article MMPs: Matrixmetalloproteinases reviews previously proposed eruption theories, in the RANKL: Receptor Activator of Nuclear factor Kappa B Ligand light of significant advances in the understanding that the sequential interactions between dental epithelium and REE: Reduced Enamel Epithelium ectomesenchymal cells pattern the initiating cascade of TNF- α: Tumour Necrosis Factor–α the eruption process. These findings are presented in the context of tooth development within the milieu of a changing bony socket. Understanding the process of tooth eruption during this phase are subdivided into intra-osseous and in this framework points to the fact that tooth eruption is supra-osseous stages referring to the movement of the essentially a stage of tooth development which, through tooth from a position within the bone to its functional po- 1 selective resorption and deposition of bone, allows the sition in occlusion. -

Preventive Treatment of Ectopically Erupting Maxillary Permanent Canines by Extraction of Deciduous Canines and First Molars: A
ORIGINAL ARTICLE Preventive treatment of ectopically erupting maxillary permanent canines by extraction of deciduous canines and first molars: A randomized clinical trial Giulio Alessandri Bonetti,a Matteo Zanarini,b Serena Incerti Parenti,c Ida Marini,b and Maria Rosaria Gattod Bologna, Italy Introduction: In this research project, we aimed to compare the effectiveness of single (1 deciduous canine) and double (deciduous canine and first molar) extractions in subjects with retained maxillary permanent canines positioned palatally or centrally in the alveolar crest, at risk for root resorption of adjacent permanent teeth. Methods: Subjects at risk for canine impaction or resorptive situations were randomly assigned to 1 of 2 treat- ment modalities: single extraction (17 patients, 28 canines) or double extraction (20 patients, 37 canines). Thirty-one patients with 53 canines judged to be not at risk constituted the untreated control group. Panoramic radiographs were taken at the initial observation and after 18 months on average. Between-group statistical comparisons were carried out on the changes in canine inclination and sector location (measured on panoramic radiographs) and on the percentages of successful permanent canine eruptions. Results: The double-extraction group showed significant improvements in the success rate and the intrabony position of the permanent canine, in terms of uprighting the canine’s long axis with a crown movement in a distal direction. Conclusions: Concomitant deciduous canine and first molar extractions proved to be more effective as a preventive approach to promote eruption of retained maxillary permanent canines positioned palatally or centrally. (Am J Orthod Dentofacial Orthop 2011;139:316-23) axillary canine impaction is often encountered in severity of the impaction and, if possible, encourage the orthodontic clinical practice1-3;thefrequency eruption of the canine, thus avoiding possible M 2 5,7,9-13 ranges from 1.7% in the general population to detrimental effects. -

The London Atlas of Human Tooth Development and Eruption
AMERICAN JOURNAL OF PHYSICAL ANTHROPOLOGY 142:481–490 (2010) Brief Communication: The London Atlas of Human Tooth Development and Eruption S.J. AlQahtani, M.P. Hector, and H.M. Liversidge* Institute of Dentistry, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London E1 2AD, UK KEY WORDS dental; age; estimation; forensic; odontology ABSTRACT The aim of this study was to develop a for all age categories was used to construct the atlas. comprehensive evidence-based atlas to estimate age Tooth development was determined according to Moor- using both tooth development and alveolar eruption for rees et al. (J Dent Res 42 (1963a) 490–502; Am J Phys human individuals between 28 weeks in utero and 23 Anthropol 21 (1963b) 205–213) and eruption was years. This was a cross-sectional, retrospective study of assessed relative to the alveolar bone level. Intraexa- archived material with the sample aged 2 years and miner reproducibility calculated using Kappa on 150 older having a uniform age and sex distribution. Devel- teeth was 0.90 for 15 skeletal remains of age <2 years, oping teeth from 72 prenatal and 104 postnatal skeletal and 0.81 from 605 teeth (50 radiographs). Age categories remains of known age-at-death were examined from col- were monthly in the last trimester, 2 weeks perinatally, lections held at the Royal College of Surgeons of Eng- 3-month intervals during the first year, and at every land and the Natural History Museum, London, UK (M year thereafter. Results show that tooth formation is 91, F 72, unknown sex 13).