Alexander Technique Exposure and Physiological Measures of Movement Planning and Execution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alexander Technique Principles & 21St Century Research
Science Catches Up – Alexander Technique Principles & 21st Century Research © 2019, Rajal G. Cohen, Ph.D. I have spent the last 18 years trying to understand Alexander technique from a scientific perspective, using insights from AT to inspire testable hypotheses, and testing those hypotheses. It is now over 100 years since FM Alexander published his first book, and scientists are finally asking questions where insights from Alexander technique can make a difference. Before examining some relevant modern research, let’s review a few key aspects of how science works. Peer Review and Replication No matter how careful and objective scientists try to be, we all need outside perspectives on our work. Peer review and replication are two of the most important parts of the scientific process. Here’s how it works: After I write an article, I submit it to a peer-reviewed journal for consideration. The editor glances at it and decides whether to reject it immediately or send it out for review. It often takes months to gather reviews from willing volunteers with suitable expertise. A paper could be accepted or rejected at this stage; more typically, reviewers will request specific changes, which might include things like ‘run another study’ or ‘re-analyze the data.’ Multiple rounds of review are common. Most published researchers also serve as reviewers, and most of us try to provide constructive feedback that will lead to a better final published paper. Any paper not published in a peer-reviewed scientific journal has not passed this essential test.1–3 Even with peer review, a single study is never enough. -

Reducing Performance Anxiety in Woodwind Playing Through the Application of the Alexander Technique Principles
i REDUCING PERFORMANCE ANXIETY IN WOODWIND PLAYING THROUGH THE APPLICATION OF THE ALEXANDER TECHNIQUE PRINCIPLES By ANNELIE HOBERG Submitted in fulfillment of the requirements for the degree of Master of Music In the Department of Music Of the Faculty of Humanities University of Pretoria Supervisor: Prof. J. de C. Hinch Pretoria April 2008 © University of Pretoria ii Abstract Many musicians are forced to scale down or even abandon their profession due to injuries caused by playing and practicing their instruments for long hours in unnatural body positions; also, the competition and the high standard expected of performers in the industry causes anxiety and tension in their lives. Increasingly, music schools, conservatories and colleges attempt to cater for these problems by including classes in the Alexander Technique. But young learners can also fall prey to these problems. Performance anxiety is potentially a threat to any musician’s career and can be experienced at all levels of expertise. In the light of this the following research question was tested: Is it possible to substantially reduce performance anxiety (stage fright) in instrumental playing by implementing Alexander Technique principles? The study included a group of twelve school- going children who had been identified as being prone to performance anxiety. They were divided into two groups of six each, one being the experimental group and the other the control group. The experimental group consisted of six flute students who were exposed to selected Alexander Technique principles in their lessons, while the control group consisted of six flute students that were taught by different teachers, who provided no exposure. -
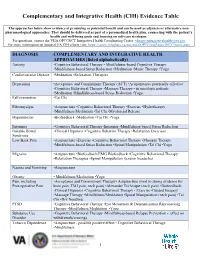
Complementary and Integrative Health (CIH) Evidence Table
Complementary and Integrative Health (CIH) Evidence Table The approaches below show evidence of promising or potential benefit and can be used as adjuncts or alternative non- pharmacological approaches. They should be delivered as part of a personalized health plan, connecting with the patient’s health and well-being goals and focusing on self-care strategies. For questions, contact the VHA OPCC&CT Integrative Health Coordinating Center: [email protected] For more information on national VA CIH efforts visit: https://vaww.infoshare.va.gov/sites/OPCC/sitePages/IHCC-home.aspx DIAGNOSIS COMPLEMENTARY AND INTEGRATIVE HEALTH APPROACHES (listed alphabetically) Anxiety •Cognitive Behavioral Therapy • Mindfulness-based Cognitive Therapy •Mindfulness-based Stress Reduction •Meditation •Music Therapy •Yoga Cardiovascular Disease •Meditation •Relaxation Therapies Depression •Acceptance and Commitment Therapy (ACT) •Acupuncture- potentially effective •Cognitive Behavioral Therapy •Massage Therapy - in oncologic patients •Meditation •Mindfulness-based Stress Reduction •Yoga Fall prevention •Tai Chi Fibromyalgia •Acupuncture •Cognitive Behavioral Therapy •Exercise •Hydrotherapy •Mindfulness Meditation •Tai Chi •Myofascial Release Hypertension •Biofeedback •Meditation •Tai Chi •Yoga Insomnia •Cognitive Behavioral Therapy-Insomnia •Mindfulness-based Stress Reduction Irritable Bowel •Clinical Hypnosis •Cognitive Behavior Therapy •Relaxation Exercises Syndrome Low Back Pain •Acupuncture •Exercise •Cognitive Behavioral Therapy •Massage Therapy -

Somatics Studies and Dance GLENNA BATSON DSC, PT, MA with the IADMS DANCE EDUCATORS’ COMMITTEE, 2009
RESOURCE PAPER FOR DANCERS AND TEACHERS Somatics Studies and Dance GLENNA BATSON DSC, PT, MA WITH THE IADMS DANCE EDUCATORS’ COMMITTEE, 2009. INTRODUCTION 2 HISTORY 2 KEY CONCEPTS 3 NOVEL LEARNING CONTEXTS 4 SENSORY ATTUNEMENT 4 AUGMENTED REST 5 SOMATIC PRACTICES IN DANCE TECHNIQUE 6 IDEOKINESIS 6 THE FELDENKRAIS METHOD® 7 ALEXANDER TECHNIQUE 8 BODY-MIND CENTERING 9 FURTHER SUBSTANTIATION 10 STUDY AND CERTIFICATION 10 FURTHER THOUGHTS 10 ADDITIONAL RESOURCES 11 REFERENCES 11 1. INTRODUCTION “I think, therefore I move” Thomas Hanna Since the 1970s, a growing number of dancers have sought additional training in mind- body techniques loosely called “somatic studies,” or simply, “somatics.”1 Once considered esoteric and far removed from daily technique class, somatics is now a household word in a dancer’s training. University dance programs worldwide now offer substantive somatic studies2 and degree programs,3 and community studios offer extensive study and certification in various practices.4,5 2. HISTORY Somatic studies also have been referred to as body therapies, bodywork, body-mind integration, body-mind disciplines, movement awareness, and movement (re) education.6 The origins of western somatic education are rooted in a philosophical revolt against Cartesian dualism.7,8 In the European Gymnastik movement of the late 19th century, for example, somatic pioneers Francois Delsarte, Emile JaquesDalcroze, and Bess Mensendieck sought to replace the reigning ideology of rigor in physical training with a more “natural” approach based on listening -

Alexander Technique
April 2014 MEDELLA, April 2014 VolumePage 1, 5Issue 3 MEDELLA CURE , HEALING POWER , H EALTH GW Center for Integrative Medicine 908 New Hampshire Avenue NW Washington D.C. 20037 Metro: Foggy Bottom Website: www.gwcim.com E-mail address: [email protected] Phone: 202-833-5055 Hours: 9 am to 5 pm Doctor of the future: INSIDE THIS ISSUE What do patients want? Doctor of the Future 1 A quote widely used on integrative medicine Alexander Technique 1 websites, with slight variations in wording, states: "The Relaxation for Insomnia 2 doctor of the future will give little medicine but will interest his patients in care of the human frame, diet, and in the cause and prevention of disease." Most websites attribute this saying to Thomas Edison, but some claim Albert Einstein said it. While it looks like doctors who understand that health and disease involve the true provenance isn't clear, it neatly sums up the the mind, body, and spirit and that health is philosophy of integrative medicine. determined in part by the context of patients’ lives. Patients value the relationship with their physician Consideration of this saying leads to the question: above all else, including service. What do patients really want from their medical providers? Are they seeking knowledge and compassion; treatment of their disease and symptoms; or an ongoing prevention, education, and health optimization program? Here is a look at several studies that have tried to answer this question. The Future of Family Medicine project, initiated in 2002 by the leadership of seven national family medicine organizations, defined family physicians as Please see Doctor on page 2 Alexander Technique: Better living through better posture As part of its monthly guest speaker series, in April the in turn helps them project their voices more GW Center for Integrative Medicine hosted Mary Naden, a powerfully. -

Noninvasive Nonpharmacological Treatment for Chronic Pain: a Systematic Review Evidence Summary
Comparative Effectiveness Review Number 209 Effective Health Care Program Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review Evidence Summary Introduction Purpose of Review Chronic pain substantially impacts physical and mental functioning, productivity, To assess which noninvasive nonpharmacological treatments for common quality of life, and family relationships; chronic pain conditions improve function and it is the leading cause of disability and is pain for at least 1 month after treatment. often refractory to treatment.1,2 Chronic pain is often defined as pain lasting 3 Key Messages months or longer or persisting past the • Interventions that improved function and/ normal time for tissue healing, though or pain for at least 1 month when used definitions vary.1,3 Chronic pain affects for— millions of adults in the United States, – Chronic low back pain: Exercise, with an annual cost in personal and health psychological therapies (primarily system expenditures conservatively cognitive behavioral therapy [CBT]), estimated at $560 billion to $635 spinal manipulation, low-level laser billion.1 Chronic pain is multifaceted therapy, massage, mindfulness-based and is influenced by multiple factors stress reduction, yoga, acupuncture, (e.g., genetic, central nervous system, multidisciplinary rehabilitation psychological, and environmental factors) (MDR). and complex interactions, making pain – Chronic neck pain: Exercise, low- assessment and management a challenge. level laser, Alexander Technique, acupuncture. Many pharmacological and – Knee osteoarthritis: Exercise, nonpharmacological treatments are ultrasound. available for management of chronic pain and include a variety of noninvasive – Hip osteoarthritis: Exercise, manual therapies. as well as surgical and interventional procedures. The National Pain Strategy – Fibromyalgia: Exercise, CBT, (NPS) report2 and 2011 Institute of myofascial release massage, tai chi, Medicine (IOM) report1 describe the qigong, acupuncture, MDR. -

Applications of Somatic Education Principles to Voice Pedagogy
Running head: SOMATIC EDUCATION AND VOICE PEDAGOGY APPLICATIONS OF SOMATIC EDUCATION PRINCIPLES TO VOICE PEDAGOGY By ALISON J. MINGLE A dissertation submitted to the Mason Gross School of the Arts Rutgers, the State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Musical Arts Graduate Program in Music Written under the direction of Dr. Stephanie Cronenberg And approved by ______________________________ Dr. Stephanie Cronenberg ______________________________ Dr. William Berz ______________________________ Professor Judith Nicosia ______________________________ Dr. Kathy Price New Brunswick, New Jersey May, 2018 SOMATIC EDUCATION AND VOICE PEDAGOGY ABSTRACT OF THE DISSERTATION Applications of Somatic Education Principles to Voice Pedagogy By ALISON J. MINGLE Dissertation Director: Dr. Stephanie Cronenberg Students of voice are trained in complex processes of coordinated movements, many of which occur internally. Somatic education techniques may promote complementary skills, such as the ability to integrate the mind and body, to move with ease and efficiency, and to intervene with awareness and volition against interfering habits. After examining three related approaches to somatic education, a multiple case study was conducted on three professional voice teachers, each of whom is also certified in the somatic education disciplines of the Alexander Technique, the Feldenkrais Method, or Hanna Somatic Education. Each teacher was interviewed three times, and observed teaching four voice lessons. One voice student at each site was interviewed as well. A comparison and analysis of interview and observation data from these contexts may illuminate the ways in which somatic education principles can be applied to the teaching of voice. ii SOMATIC EDUCATION AND VOICE PEDAGOGY For Nana Barbara. -

Complementary and Alternative Medicine
THE ENCYCLOPEDIA OF COMPLEMENTARY AND ALTERNATIVE MEDICINE THE ENCYCLOPEDIA OF COMPLEMENTARY AND ALTERNATIVE MEDICINE Tova Navarra, B.A., R.N. Foreword by Adam Perlman, M.D., M.P.H. Siegler Center for Integrative Medicine St. Barnabas Health Care System, Livingston, New Jersey The Encyclopedia of Complementary and Alternative Medicine Copyright © 2004 by Tova Navarra All rights reserved. No part of this book may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage or retrieval systems, without permission in writing from the publisher. For information contact: Facts On File, Inc. 132 West 31st Street New York NY 10001 Library of Congress Cataloging-in-Publication Data Navarra, Tova The encyclopedia of complementary and alternative medicine / Tova Navarra; foreword by Adam Perlman. p.cm. Includes bibliographical references and index. ISBN 0-8160-4997-1 1. Alternative medicine—Encyclopedias. I. Title. R733. N38 2004 615.5'03—dc21 2003043415 Facts On File books are available at special discounts when purchased in bulk quantities for businesses, associations, institutions, or sales promotions. Please call our Special Sales Department in New York at (212) 967-8800 or (800) 322-8755. You can find Facts On File on the World Wide Web at http://www.factsonfile.com Text and cover design by Cathy Rincon Printed in the United States of America VB FOF 10 9 8 7 6 5 4 3 2 1 This book is printed on acid-free paper. For Frederic CONTENTS Foreword ix Preface xiii Acknowledgments xv Introduction xvii Entries A–Z 1 Appendixes 175 Bibliography 251 Index 255 FOREWORD t the age of 16 I began training in martial arts. -

Alexander Technique Vs. Targeted Exercise for Neck Pain—A Preliminary Comparison
applied sciences Article Alexander Technique vs. Targeted Exercise for Neck Pain—A Preliminary Comparison Jordan J. Becker 1,2, Tara L. McIsaac 3 , Shawn L. Copeland 4 and Rajal G. Cohen 1,* 1 Department of Psychology & Communication, University of Idaho, Moscow, ID 83844, USA; [email protected] 2 National Center of Complementary and Integrative Health, National Institutes of Health, Bethesda, MD 20892, USA 3 Department of Physical Therapy, A.T. Still University, Mesa, AZ 85206, USA; [email protected] 4 Lionel Hampton School of Music, University of Idaho, Moscow, ID 83844, USA; [email protected] * Correspondence: [email protected] Abstract: Background: Alexander technique private lessons have been shown to reduce chronic neck pain and are thought to work by different mechanisms than exercise. Group classes may also be effective and would be cost-effective. Design: A two-group pre-test/post-test design. Participants were assigned to either a general Alexander technique class or an exercise class designed to target neck pain. Both groups met over 5 weeks for two 60 min sessions/week. Participants: A total of 16 participants with chronic neck pain (aged 50+/−16 years) completed this study. Interventions: The Alexander class used awareness-building methods to teach participants to reduce habitual tension during everyday activities. The exercise class was based on physical therapy standard of care to strengthen neck and back muscles thought to be important for posture. Measures: We assessed neck pain/disability, pain self-efficacy, activation of the sternocleidomastoid muscles during the cranio-cervical flexion test, and posture while participants played a video game. -

Mind-Body Practices in Integrative Medicine
Religions 2012, 3, 50–81; doi:10.3390/rel3010050 OPEN ACCESS religions ISSN 2077-1444 www.mdpi.com/journal/religions Article Mind-Body Practices in Integrative Medicine Harald Walach 1,2, Marie-Louise Gander Ferrari 3, Sebastian Sauer 4 and Niko Kohls 2,4,* 1 Institute of Transcultural Health Studies, European University Viadrina, Postfach 1786, D-15207 Frankfurt (Oder), Germany; E-Mail: [email protected] 2 Brain, Mind and Healing Program, Samueli Institute, 1737 King Street, Suite 600, Alexandria, VA 22314, USA 3 Department of General Internal Medicine, Division of Psychosomatic Medicine, Inselspital, Bern University Hospital, CH-3010 Bern, Switzerland; E-Mail: [email protected] 4 Generation Research Program, Human Science Center, Ludwig-Maximilians-Universität, Prof.-Max-Lange-Platz 11, 83646 Bad Tölz, Germany * Author to whom correspondence should be addressed; E-Mail: [email protected]. Received: 3 February 2012; in revised form: 11 February 2012 / Accepted: 21 February 2012 / Published: 23 February 2012 Abstract: Mind-Body practices have become increasingly popular as components of psychotherapeutic and behavior medicine interventions. They comprise an array of different methods and techniques that use some sort of mental-behavioral training and involve the modulation of states of consciousness in order to influence bodily processes towards greater health, well-being and better functioning. Mind-body practices may thus be interpreted as the salutogenetic mirror image of psychosomatic medicine, where psychophysiological and health consequences of specific psychological states are studied, such as stress arousal, psychological trauma or depression. This contribution examines the empirical evidence of the most common mind-body techniques with regard to their salutogenetic potential. -

Influence of Mechanical Choices on Development and Persistence of Osteoarthritis: How Alexander Technique Can Promote Prevention and Management Rachelle E
East Tennessee State University Digital Commons @ East Tennessee State University Undergraduate Honors Theses Student Works 5-2016 Influence of Mechanical Choices on Development and Persistence of Osteoarthritis: How Alexander Technique Can Promote Prevention and Management Rachelle E. Lowry East Tennessee State Universtiy Follow this and additional works at: https://dc.etsu.edu/honors Part of the Movement and Mind-Body Therapies Commons Recommended Citation Lowry, Rachelle E., "Influence of Mechanical Choices on Development and Persistence of Osteoarthritis: How Alexander Technique Can Promote Prevention and Management" (2016). Undergraduate Honors Theses. Paper 351. https://dc.etsu.edu/honors/351 This Honors Thesis - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Undergraduate Honors Theses by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. Influence of Mechanical Choices on Development and Persistence of Osteoarthritis: How Alexander Technique Can Promote Prevention and Management by Rachelle Elizabeth Lowry An Honors Thesis submitted in partial fulfillment of the requirements of the Fine and Performing Arts Honors Scholars Program, the Honors College, and the Department of Health Sciences, College of Public Health East Tennessee State University April 15, 2016 0 | P a g e Table of Contents Abstract ...................................................................................................................................................... -

Potential Mechanisms of the Alexander Technique: Toward a Comprehensive Neurophysiological Model
Kinesiology Review, (Ahead of Print) https://doi.org/10.1123/kr.2020-0026 © 2020 Human Kinetics, Inc. SCHOLARLY ARTICLE Potential Mechanisms of the Alexander Technique: Toward a Comprehensive Neurophysiological Model Timothy W. Cacciatore, Patrick M. Johnson, and Rajal G. Cohen The Alexander technique (AT) has been practiced for over 125 years. Despite evidence of its clinical utility, a clear explanation of how AT works is lacking, as the foundational science needed to test the underlying ideas has only recently become available. The authors propose that the core changes brought about by Alexander training are improvements in the adaptivity and distribution of postural tone, along with changes in body schema, and that these changes underlie many of the reported benefits. They suggest that AT alters tone and body schema via spatial attention and executive processes, which in turn affect low-level motor elements. To engage these pathways, AT strategically engages attention, intention, and inhibition, along with haptic communication. The uniqueness of the approach comes from the way these elements are woven together. Evidence for the contribution of these elements is discussed, drawing on direct studies of AT and other relevant modern scientific literature. Keywords: body axis, body schema, muscle tone, musculoskeletal pain, postural tone, somatic practice Evidence is mounting that practicing the Alexander technique mechanisms of AT have been published (Becker, Copeland, (AT) has a range of benefits. Clinical research suggests that it can Botterbusch, & Cohen, 2018; Cacciatore, Gurfinkel, Horak, help alleviate common musculoskeletal complaints such as chronic Cordo, & Ames, 2011; Cacciatore, Gurfinkel, Horak, & Day, back, neck, and knee pain (Little et al., 2008; MacPherson et al., 2011; Cacciatore et al., 2014; Cohen et al., 2015, 2020; Hamel 2015; Preece, Jones, Brown, Cacciatore, & Jones, 2016).