Balance and Breadth And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Expect the Unexpected: Building Business Value in a Changing World
Expect the Unexpected: Building business value in a changing world kpmg.com KPMG INTERNATIONAL PART 1 b | Expect the Unexpected: Building business value in a changing world In this report we offer a starting point for discussion. We present a system of ten sustainability megaforces that will impact each and every business over the next 20 years. We want to build awareness that these forces do not act alone in predictable ways. They are interconnected. They interact. Disclaimer: Throughout this document, "KPMG" ["we," "our," and "us"] refers to KPMG International, a Swiss entity that serves as a coordinating entity for a network of independent member firms operating under the KPMG name, KPMG's Climate Change and Sustainability practice, and/or to any one or more of such firms. KPMG International provides no client services. © 2012 KPMG International Cooperative (“KPMG International”), a Swiss entity. Member firms of the KPMG network of independent firms are affiliated with KPMG International. KPMG International provides no client services. All rights reserved. Expect the Unexpected: Building business value in a changing world | c Foreword usinesses today are operating in an ever more At KPMG’s network of firms we have always been at the interconnected and globalized world. Supply chains forefront of developments that shape business behavior. Bstretch across continents and are vulnerable to We are working with organizations to help them understand disruption. Consumer demands and government policies the forces at work that will influence markets and impact are changing rapidly and will impact your bottom line if your profitability in the medium to long term. -

2015 Informa Investor Day
2015 Informa Investor Day 18 November 2015 INTRODUCTION STEPHEN A. CARTER GROUP CHIEF EXECUTIVE 2015 Informa Investor Day Presentation Schedule • Introduction • Academic Publishing – Content Depth and Operating Efficiency • Global Exhibitions – Maximising the Growth Opportunity • Greenbuild – Overview & Tour • Lunch • Operational & Financial Update • Summary and Q&A THE JOURNEY SO FAR Stephen Carter appointed 2014 Investor Day Group CEO & launch of Disposal of Consumer Info. strategic review Review of strategic options for European Conferences Gareth Wright confirmed as Group FD, external appointment of BI and K&N Dwell acquisition FIME acquisition CEO’s 2013 2016 2014-2017 Growth Hanley Wood Disposal of Acceleration Plan Exhibitions acquisition Scandinavian announced and placing Conferences New operating Virgo acquisition Orlando Megacon Disposal of Dutch structure announced acquisition Conferences INFORMA GROUP INVESTOR DAY NOVEMBER 2015 4 2014-2017 GROWTH ACCELERATION PLAN FRAMEWORK Growth Acceleration Plan GAP GAP GAP GAP GAP GAP Operating Management Portfolio Acquisition Investment Funding Structure Model Management Strategy • 4 Operating Divisions • EMT established • Consumer Info disposal • Hanley Wood Exhib. • £70-90m programme • Renegotiated RCF • Global Support • Divisional SMTs • InsuranceIQ disposal • Virgo • 30-40 projects • $250m PPP issue • Medical Journals transfer • GE succession • Chinese Pharma closure • Orlando Megacon • 21 projects underway • Group Treasurer • WPA and Learning transfer • 3 yr business plans • Closure -

Novartis Swaps Two Avexis Executives for One Following
No. 3969 August 23, 2019 and CSO that the Kaspars have not been involved in the Novartis subsidiary’s op- erations since early May. Asked whether more AveXis employees will exit the company due to the preclini- cal safety data manipulation revealed on 6 August or whether other actions will be taken by Novartis in relation to the matter, Althoff told Scrip: “We cannot comment on the specifics of who is involved, but we can confirm this issue was not widespread, ei- ther in our overall dataset nor in our com- pany or any function within our company and was limited to a few individuals.” Novartis CEO Vas Narasimhan said in a 7 August conference call about the Zol- gensma data controversy that the compa- ny was in the process of “exiting the small number of AveXis scientists involved” in the data manipulation, which occurred Novartis Swaps Two AveXis before Novartis bought the gene therapy firm. (Also see “Novartis CEO Explains De- lay In Telling US FDA About Zolgensma Executives For One Following Data Fraud: We Wanted To Understand It First” - Pink Sheet, 7 Aug, 2019.) Zolgensma Data Manipulation Novartis acquired AveXis for $8.7bn MANDY JACKSON [email protected] last year. (Also see “Novartis Goes Big On Gene Therapy With $8.7bn AveXis Acquisi- ovartis AG said when it disclosed the Zolgensma BLA was manipulated. Page tion” - Scrip, 9 Apr, 2018.) Zolgensma was that preclinical safety data for Zol- Bouchard, whose most recent function was the lead asset in AveXis’s R&D pipeline and Ngensma were manipulated in the bi- global head of preclinical safety for Novar- won US Food and Drug Administration ologic license application for the gene ther- tis Institutes of Biomedical Research (NIBR), approval in May, becoming the world’s apy that the company would be “exiting” was named senior vice president and chief most expensive drug with a $2m list price. -

Visualizing B Cell Development: Creating an Immunology Video Game
VISUALIZING B CELL DEVELOPMENT: CREATING AN IMMUNOLOGY VIDEO GAME By Emily Lunhui Ling A thesis submitted to Johns Hopkins University in conformity with the requirements for the degree of Master of Arts Baltimore, Maryland March, 2016 © 2016 Emily L. Ling All Rights Reserved ABSTRACT e foundational immunology concepts of lymphocyte development are important for beginning science students to comprehend. Video games oer the potential for a novel approach to teaching this complex subject matter by more eectively engaging students in this material. However, currently available educational video games intended to teach immunology have distinct limitations such as a lack of explicit demonstrations of the stages of lymphocyte development and clonal selection. is project identies the content focus and gameplay mechanics of currently available immunology video games. Using this as a basis, a novel approach for developing an immunology video game was outlined with the primary goal of improving integration of educational content. A proof of concept was developed for the B lymphocyte development portion of the game content and a partial prototype was developed in Unity 5 3D. e important contribution of this thesis was the development of a new approach to designing a more eective educational video game specically for immunology. Outcomes of this research will serve to inform future biomedical communicators on how to develop content for active learning games in immunology and provide a guide for designing full length educational video games featuring novel gameplay mechanics such as those identied through this project. Emily L. Ling ii CHAIRPERSONS OF THE SUPERVISORY COMMITTEE esis Preceptor Mark J. Soloski, Ph.D., Professor of Medicine Departments of Medicine, Pathology, Molecular Biology and Genetics, and Molecular Microbiology and Immunology Director, Immunology Training Program e Johns Hopkins University School of Medicine Departmental Advisor David A. -

Informa 2018 Full Year Results Statement
Informa LEI: 5493006VM2LKUPSEDU20 Press Release 7 March 2019 Informa PLC Results for 12 Months to 31 December 2018 2018: Combination & Creation 2019: Performance & Growth KEY FINANCIAL AND OPERATING HIGHLIGHTS1 • Strong Revenue Growth: +3.7% underlying and +34.9% reported to £2,369.5m, including six months of UBM (2017: £1,756.8m) • Higher Adjusted Operating Profit: +2.3% underlying and +34.4% reported to £732.1m (2017: £544.9m) • Improved Statutory Operating Profit: £363.2m (2017: £344.7m) • Increased Adjusted Diluted Earnings per Share: +7.0% to 49.2p (2017: 46.0p); Statutory EPS of 19.7p (2017: 37.6p), with prior year including non-cash credit from US tax reforms • Attractive Free Cash Flow: £503.2m and £600m+ including a full year of UBM (2017: £400.9m) • Robust Balance Sheet, in line with plan: Net debt/EBITDA1 at 2.9x (2017: 2.5x) • Enhanced Dividend: up 7.1% to 21.90p (2017: 20.45p) London: Informa (LSE: INF.L), the International Exhibitions, Events, Information Services and Scholarly Research Group, today published its financial results for the 12 months to 31 December 2018, reporting a further period of operational progress and improving financial performance. Stephen A. Carter, Group Chief Executive, said: “In 2018, the Informa Group delivered a fifth consecutive year of improving growth, increasing adjusted profits, adjusted earnings per share, cashflow and dividends.” He added: “In 2019, our focus is on continuing Performance and Growth as we consolidate our market positions and further reduce complexity. This will enable -
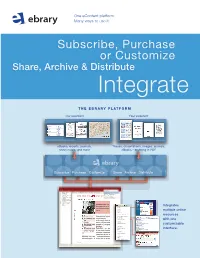
The EBRARY Platform
One eContent platform. Many ways to use it. Subscribe, Purchase or Customize Share, Archive & Distribute Integrate THE EBRARY PLatFORM Our eContent Your eContent eBooks, reports, journals, Theses, dissertations, images, journals, sheet music, and more eBooks – anything in PDF Subscribe Purchase Customize Share Archive Distribute SULAIR Select Collections SUL EBRARY COLLECTIONS Select Collections TABLE OF CONTENTS Infotools All ebrary Collections CONTRIBUTORS Byron Hoyt Sheet Music (Browse) INTRODUCTION Immigration Commision Reports (Dillingham) Contents BUSINESS: A USER’S GUIDE Women and Child Wage Earners in the U.S. CSLI Linguistics and Philosophy BEST PRACTICE SUL Books in the Public Domain MANAGEMENT CHECKLISTS Medieval and Modern Thought Text Project ACTIONLISTS MANAGEMENT LIBRARY BUSINESS THINKERS AND MANA DICTIONARY WORLD BUSINESS ALMANAC Highlights Notes BUSINESS INFORMATION SOURC INDEX CREDITS InfoTools Integrates Define Explain Locate multiple online Translate Search Document... resources Search All Documents... Search Web Search Catalog with one Highlight Add to Bookshelf customizable Copy Text... Copy Bookmark interface. Print... Print Again Toggle Automenu Preferences... Help About ebrary Reader... EASY TO USE. Subscribe, Purchase AFFORDABLE. or Customize your ALW ay S AVAILABLE. eBook selection NO CHECK-OUTS. Un I Q U E S U B SC R I B E T O G R OWI ng E B O O K D ataba S E S W I T H SIMULtanEOUS, MULTI-USER ACCESS. RESEARCH TOOLS. ACADEMIC DATABASES Academic Complete includes all academic databases listed below. FREE MARCS. # of # of Subject Titles* Subject Titles* Business & Economics 6,300 Language, Literature 3,400 & Linguistics “ebrary’s content is Computers & IT 2,800 Law, International Relations 3,800 multidisciplinary and Education 2,300 & Public Policy Engineering & Technology 3,700 supports our expanding Life Sciences 2,000 (includes Biotechnolgy, History & Political Science 4,800 Agriculture, and (also includes a bonus selection faculty and curriculum. -

Annual Copyright License for Corporations
Responsive Rights - Annual Copyright License for Corporations Publisher list Specific terms may apply to some publishers or works. Please refer to the Annual Copyright License search page at http://www.copyright.com/ to verify publisher participation and title coverage, as well as any applicable terms. Participating publishers include: Advance Central Services Advanstar Communications Inc. 1105 Media, Inc. Advantage Business Media 5m Publishing Advertising Educational Foundation A. M. Best Company AEGIS Publications LLC AACC International Aerospace Medical Associates AACE-Assn. for the Advancement of Computing in Education AFCHRON (African American Chronicles) AACECORP, Inc. African Studies Association AAIDD Agate Publishing Aavia Publishing, LLC Agence-France Presse ABC-CLIO Agricultural History Society ABDO Publishing Group AHC Media LLC Abingdon Press Ahmed Owolabi Adesanya Academy of General Dentistry Aidan Meehan Academy of Management (NY) Airforce Historical Foundation Academy Of Political Science Akademiai Kiado Access Intelligence Alan Guttmacher Institute ACM (Association for Computing Machinery) Albany Law Review of Albany Law School Acta Ecologica Sinica Alcohol Research Documentation, Inc. Acta Oceanologica Sinica Algora Publishing ACTA Publications Allerton Press Inc. Acta Scientiae Circumstantiae Allied Academies Inc. ACU Press Allied Media LLC Adenine Press Allured Business Media Adis Data Information Bv Allworth Press / Skyhorse Publishing Inc Administrative Science Quarterly AlphaMed Press 9/8/2015 AltaMira Press American -

Global Business Information Specialist Informa’S Book Business Has More Than 55,000 Academic Advisers 26 Directors’ Report 27 and Business Titles
Business Profile Business WHO WE ARE Business Profile 02 Annual Report & Financial Statements 2009 Our Business 03 Informa at a Glance 04 Academic Information 06 Professional & Commercial Information 08 Events & Training 10 Informa plc is a leading international provider of specialist information and services for the academic and scientific, professional and commercial business communities. Informa has some 150 offices in over 40 countries and employs approximately 8,000 staff around the world. Informa is the largest publicly-owned organiser of conferences and courses in the world with inThe Year The Year in Review 12 an output of around 8,000 events annually. Informa Financial Highlights 13 publishes over 2,100 subscription-based information Chairman’s Statement 14 services including academic journals, real-time news Chief Executive’s Review 16 Financial Review 19 R and structured databases of commercial intelligence. Board of Directors 24 eview Global Business Information Specialist Informa’s book business has more than 55,000 academic Advisers 26 Directors’ Report 27 and business titles. Corporate Governance Statement 38 Directors’ Remuneration Report 45 Corporate Responsibility 55 Financial Statements Financial Financial Statements 58 Independent Auditors’ Report – Group 59 Consolidated Income Statement 60 Consolidated Statement of Comprehensive Income 61 Consolidated Statement of Changes in Equity 62 Consolidated Statement of Financial Position 64 ”It was an extremely demanding year, Consolidated Cash Flow Statement 65 but one which -

Informa Corporate Structure
Corporate Structure From www.informa.com/Who-We-Are/Corporate-structure/ 5 March 2011 History Edward Lloyd pins the first ever copy of his shipping list to the wall of his coffee shop 1734 in Lombard Street, City of London. Richard Taylor publishes the first edition of The Philosophical Magazine, one of the 1798 first scientific journals produced by an independent company Dr William Francis joined Richard Taylor to found Taylor & Francis and continue the 1852 close links between the academic community and the company Taylor & Francis became a private limited company with leading scientists as 1936 directors and shareholders 1964 Investment & Property Studies founded as a part-time business International Business Communications Ltd (IBC) created to be an umbrella company 1971 for Investment & Property Studies and Legal Studies & Services The Institute for International Research (IIR) is launched by Irvine Laidlaw later to 1973 become Baron Laidlaw of Rothiemay – IIR becomes a specialist business conference organiser 1976 Euroforum established in Holland by IBC 1985 International Business Communications (Holdings) plc established Datamonitor is founded in London and produces its first ever report on the UK frozen 1989 food industry 1991 Launch of The Monaco Yacht Show by IIR Lloyd's List Publishing (LLP) and IBC Group plc merge to form the Informa Group 1998 plc Taylor & Francis successfully launched on the London Stock Exchange and shortly 1998 afterwards acquired the Routledge Group of companies Datamonitor, now a world-leading provider -

Characterising Action Potential in Virtual Game Worlds Applied with the Mind Module
Characterising Action Potential in Virtual Game Worlds Applied With the Mind Module Mirjam Palosaari Eladhari A thesis submitted in partial fulfilment of the requirements of the University of Teesside for the degree of Doctor of Philosophy. This research programme was carried out at and with the support of Högskolan på Gotland (Gotland University) Characterising Action Potential in Virtual Game Worlds Applied With the Mind Module Mirjam Palosaari Eladhari Gotland University Press 6 Author: Mirjam Palosaari Eladhari Publisher: Gotland University Press 2011 Address: Gotland University S-62156 Visby Web: www.hgo.se Phone: +46(0)498-29 99 00 ISSN: 1653-7424 ISBN: 978-91-86343-02-6 Editorial committee: Åke Sandström and Lena Wikström Cover: Daniel Olsson and Lena Wikström Cover illustrations: Back: Mirjam P Eladhari after details in the triptych The Garden of Earthly Delights (~1500) painted by Hieronymus Bosch. Front: The illustration The Philosophical Brain first appeared in the book ”Utriusque cosmi maioris scilicet et minores metaphysica, physica atque technica historia” (1617) by Robert Fludd, Illustrated by Matthieu Merian. Declaration While registered as a candidate for the degree of Doctor of Philosophy the author has not been registered for any other award with any other university or institution. No part of the material in this thesis has been submitted for any degree or other qualification at any other institution by the author or, to the best of her knowledge and belief, by any other person. The thesis describes the author’s original work. Other persons assisted in transcribing interviews, conducting experiments and proof reading. ii Abstract Because games set in persistent virtual game worlds (VGWs) have massive numbers of players, these games need methods of characterisation for playable characters (PCs) that differ from the methods used in traditional narrative media. -

Taylor & Francis India
Name of the Tool Taylor & Francis India Home Page Logo URL https://www.routledge.com/india Subject Bibliography Accessibility Free Language English Publisher Taylor & Francis Group Brief History The company was founded in 1852 when William Francis joined Richard Taylor in his publishing business. Taylor initially founded his company in 1798. Their subjects covered include agriculture, chemistry, education, engineering, geography, law, mathematics, medicine, and social sciences. From 1917 to 1930 Francis' son, Richard Taunton Francis (1883–1930) was sole partner in the firm. In 1965 Taylor & Francis launched Wykeham Publications and began book publishing. In 1988 it acquired Hemisphere Publishing and the company was renamed Taylor & Francis Group to reflect the growing number of imprints. The journals and e-books have been delivered through the Taylor & Francis Online website since June 2011. Scope and Coverage It is one of the world’s leading publishers of scholarly journals, books, e- books, and reference works. It covers all areas of the humanities, social sciences, behavioral sciences, science, technology and medicine. It publish more than 2,600 journals and over 5,500 new books each year, with a books backlist in excess of 120,000 specialist titles in 40 subject categories. They publish Social Science and Humanities books under the Routledge, Psychology Press and Focal Press imprints. Science, Technology and Medical books are published by CRC Press and Garland Science. Kind of Information Within an entry detail bibliographic information includes title, author, ISBN/ISSN, paginations, table of content, description, cover page image of that particular entry present here. Related items of title also available here. -

Independent Auditor's Report to the Members of Informa
Financial Statements Independent Auditor’s report to the members of Informa PLC Independent Auditor’s report to the members of Informa PLC Report on the audit of the financial statements The financial reporting framework that has been applied in Opinion the preparation of the Group financial statements is applicable In our opinion: law and IFRSs as adopted by the European Union. The financial reporting framework that has been applied in the preparation • the financial statements of Informa PLC and its of the parent company financial statements is applicable law subsidiaries (the “Group”) give a true and fair view of and United Kingdom Accounting Standards, including FRS 102 the state of the Group’s and of the parent company’s “The Financial Reporting Standard applicable in the UK and affairs as at 31 December 2018 and of the Group’s profit Republic of Ireland” (United Kingdom Generally Accepted for the year then ended; Accounting Practice). • the Group financial statements have been properly prepared in accordance with International Financial Reporting Basis for opinion Standards (IFRSs) as adopted by the European Union; We conducted our audit in accordance with International • the parent company financial statements have been properly Standards on Auditing (UK) (ISAs (UK)) and applicable law. prepared in accordance with United Kingdom Generally Our responsibilities under those standards are further Accepted Accounting Practice, including FRS 102 “The described in the auditor’s responsibilities for the audit Financial Reporting Standard applicable in the UK and of the financial statements section of our report. Republic of Ireland”; and • the financial statements have been prepared in accordance We are independent of the Group and the parent company in with the requirements of the Companies Act 2006 and, accordance with the ethical requirements that are relevant to as regards the Group financial statements, Article 4 of our audit of the financial statements in the UK, including the the IAS Regulation.