Investigating the Transport and Fate of Nitrogen from Farms to River in the Lower Rangitikei Catchment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rangitikei District Council Assets/Infrastructure Committee Meeting Order Paper — Thursday 14 July 2016 9:30 A.M
Rangitikei District Council Assets/Infrastructure Committee Meeting Order Paper — Thursday 14 July 2016 9:30 a.m. Contents 1 Welcome 2 2 Council Prayer 2 3 Apologies/Leave of absence 2 4 Confirmation of Order of business 2 5 Chair's report 2 To be tabled 6 Confirmation of minutes 2 Attachment 1, page(s) 9-18 7 Queries raised at previous meeting(s) • 2 Agenda note 8 Activity management 2 Attachment 2, page(s) 19-41 9 Emergency Works Update, June 2016— roading structures 3 Attachment 3, page(s) 42-44 10 LED streetlight replacement program 3 Attachment 4, page(s) 45-52 11 Petition from Whangaehu residents to improve safety of entrances/exits to the village 3 Attachment 5, page(s) 53-59 12 Reinstatement of heavy trailer parking near Wyleys Bridge 4 Agenda note 13 Requested signage change on SH1 for Mangaweka 4 Agenda note 14 Resource consent compliance update 4 Attachment 6, page(s) 60-70 15 Renewal of Marton wastewater treatment Plant — Update 4 Attachment 7, page(s) 71-74 Attachment 8, page(s) 16 Extended weekend hours trial — Marton Waste Transfer Station 4 75-80 Attachment 9, page(s) 17 Taihape Town Hall heating 5 81-84 18 Swim 4-All, 2015/16 5 Attachment 10, page(s) 85-91 19 Marton Park Management Plan — Draft for public consultation 6 Attachment 11, page(s) 92-112 20 Centennial Park — issues raised in submissions to 2016-17 Annual Plan 6 Agenda note 21 Proposed sale of Council-owned properties in Bulls 6 Agenda note 22 Customer satisfaction levels from Residents Survey 2016: Assets and Infrastructure 6 Attachment 12, page(s) 113-128 23 Late items 7 24 Future items for the agenda 7 25 Next meeting 7 26 Meeting closed 7 The quorum for the Assets/Infrastructure Committee is 5. -
Schedule D Part3
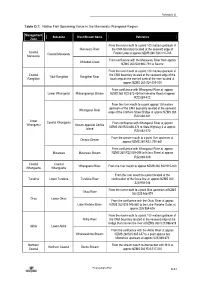
Schedule D Table D.7: Native Fish Spawning Value in the Manawatu-Wanganui Region Management Sub-zone River/Stream Name Reference Zone From the river mouth to a point 100 metres upstream of Manawatu River the CMA boundary located at the seaward edge of Coastal Coastal Manawatu Foxton Loop at approx NZMS 260 S24:010-765 Manawatu From confluence with the Manawatu River from approx Whitebait Creek NZMS 260 S24:982-791 to Source From the river mouth to a point 100 metres upstream of Coastal the CMA boundary located at the seaward edge of the Tidal Rangitikei Rangitikei River Rangitikei boat ramp on the true left bank of the river located at approx NZMS 260 S24:009-000 From confluence with Whanganui River at approx Lower Whanganui Mateongaonga Stream NZMS 260 R22:873-434 to Kaimatira Road at approx R22:889-422 From the river mouth to a point approx 100 metres upstream of the CMA boundary located at the seaward Whanganui River edge of the Cobham Street Bridge at approx NZMS 260 R22:848-381 Lower Coastal Whanganui From confluence with Whanganui River at approx Whanganui Stream opposite Corliss NZMS 260 R22:836-374 to State Highway 3 at approx Island R22:862-370 From the stream mouth to a point 1km upstream at Omapu Stream approx NZMS 260 R22: 750-441 From confluence with Whanganui River at approx Matarawa Matarawa Stream NZMS 260 R22:858-398 to Ikitara Street at approx R22:869-409 Coastal Coastal Whangaehu River From the river mouth to approx NZMS 260 S22:915-300 Whangaehu Whangaehu From the river mouth to a point located at the Turakina Lower -

WELCOME to This First Issue of Talk up Taihape for 2019! We Are Already
WELCOME to this first issue of Talk Airforce….More to follow! Finally, Up Taihape for 2019! We are we are hosting the World Boot already into the second month of Throwing Championships which are the year and have seen some great being organized by the New Zea- events in Taihape. land Boot Throwing Association The A&P show in January was a (NZBTA). This event is open to all good event and the weather played (more information on page 6) and ball. The kids had fun with all the attracting both international & activities available and the domestic media attention. shearing competitions provided great entertainment. The Waitangi Celebrations in February were held at Memorial Park and even though the temperature had dropped, the festivities were a success. We’re also very excited about Gumboot Day in March, mark it in your calendar, Saturday the 23rd from 10am till 3pm. Every year we strive to make it BIGGER & BRIGHT- This year’s sponsors for the Taihape ER than ever before and we are Gumboot Day® Family Festival certainly on our way to achieve include our Gold Sponsor: that this year. We have an Arts and Palmerston North Airport, who are Crafts Market, Bouncy Castles for running the 'Fly Palmy Have a Go all ages, and workshops where you Gumboot Throwing Competition'. are the participant. We also have Our Silver Sponsor is Byfords demonstrations, which are great to Construction 2014 Ltd and our watch, glorious food stalls, and a Bronze Sponsor is Matt Hobbs host of support from local groups, Plumbing & Drainlaying Ltd. -

NEW ZEALAND GAZR'l*IE
No. 108 2483 THE NEW ZEALAND GAZR'l*IE Published by Authority WELLINGTON: THURSDAY, 31 OCTOBER 1974 Land Taken for the Auckland-Hamilton Motorway in the SCHEDULE City of Auckland NORTH AUCKlAND LAND DISTRICT ALL that piece of land containing 1 acre 3 roods 18.7 DENIS BLUNDELL, Governor-General perches situated in Block XIII, Whakarara Survey District, A PROCLAMATION and being part Matauri lHlB Block; as shown on plan PURSUANT to the Public Works Act 1928, I, Sir Edward M.O.W. 28101 (S.O. 47404) deposited in the office of the Denis Blundell, the Governor-General of New Zealand, hereby Minister of Works and Development at Wellington and proclaim and declare that the land first described in the thereon coloured blue. Schedule hereto and the undivided half share in the land Given under the hand of His Excellency the Governor secondly therein described, held by Melvis Avery, of Auck General and issued under the Seal of New Zealand, land, machinery inspector, are hereby taken for the Auckland this 23rd day of October 1974. Hamilton Motorway. [Ls.] HUGH WATT, Minister of Works and Development. SCHEDULE Goo SAVE THE QUEEN! NORTH AUCKLAND LAND DISTRICT (P.W. 33/831; Ak. D.O. 50/15/14/0/47404) ALL those pieces of land situated in the City of Auckland described as follows: A. R. P. Being Land Taken for Road and for the Use, Convenience, or 0 0 11.48 Lot 1, D.P. 12014. Enjoyment of a Road in Blocks Ill and VII, Te Mata 0 0 0.66 Lot 2, D.P. -

Riparian Sites of Significance Based on the Habitat Requirements of Selected Bird Species : Technical Report to Support Policy Development
MANAGING OUR ENVIRONMENT Riparian Sites of Signifi cance Based on the Habitat Requirements of Selected Bird Species : Technical Report to Support Policy Development Riparian Sites of Significance Based on the Habitat Requirements of Selected Bird Species : Technical Report to Support Policy Development April 2007 Author James Lambie Research Associate Internally Reviewed and Approved by Alistair Beveridge and Fleur Maseyk. External Review by Fiona Bancroft (Department of Conservation (DoC)) and Ian Saville (Wrybill Birding Tours). Acknowledgements to Christopher Robertson (Ornithological Society of New Zealand), Nick Peet (DoC), Viv McGlynn (DoC), Jim Campbell (DoC), Nicola Etheridge (DoC), Gillian Dennis (DoC), Bev Taylor (DoC), John Mangos (New Zealand Defence Force), and Elaine Iddon (Horizons). Front Cover Photo Royal Spoonbill on Whanganui River tidal flats Photo: Suzanne Lambie April 2007 ISBN: 1-877413-72-0 Report No: 2007/EXT/782 CONTACT 24hr Freephone 0508 800 800 [email protected] www.horizons.govt.nz Kairanga Palmerston North Dannevirke Cnr Rongotea & 11-15 Victoria Avenue Weber Road, P O Box 201 Kairanga-Bunnythorpe Rds Private Bag 11 025 Dannevirke 4942 Palmerston North Manawatu Mail Centre Palmerston North 4442 Levin 11 Bruce Road, P O Box 680 Marton T 06 952 2800 Levin 5540 Hammond Street F 06 952 2929 SERVICE REGIONAL P O Box 289 DEPOTS Pahiatua CENTRES Marton 4741 HOUSES Cnr Huxley & Queen Streets Wanganui P O Box 44 181 Guyton Street Pahiatua 4941 Taumarunui P O Box 515 34 Maata Street Wanganui Mail Centre Taihape P O Box 194 Wanganui 4540 Torere Road, Ohotu Taumarunui 3943 F 06 345 3076 P O Box 156 Taihape 4742 EXECUTIVE SUMMARY The riparian zone represents a gradation of habitats influenced by flooding from a nearby waterway. -

KI UTA, KI TAI NGĀ PUNA RAU O RANGITĪKEI Rangitīkei Catchment Strategy and Action Plan 2 TABLE of CONTENTS
KI UTA, KI TAI NGĀ PUNA RAU O RANGITĪKEI Rangitīkei Catchment Strategy and Action Plan 2 TABLE OF CONTENTS STRATEGY & ACTION PLAN 4 MIHI 6 INTRODUCTION 8 THE RANGITĪKEI 14 VISION 22 5.1 Our vision 23 5.2 Ngā Tikanga | Our Values 23 5.3 Our Strategic Goals & Objectives 24 5.3.1 Te Taiao 27 5.3.2 Our Wellbeing 28 5.3.3 Our Future 29 RANGITĪKEI ACTION PLAN 31 6.1 Te Taiao 32 6.2 Our Wellbeing 39 6.3 Our Future 40 GLOSSARY 46 TOOLKIT 49 OUR LOGO 54 3 1. STRATEGY & ACTION PLAN He tuaiwi o te rohe mai i te mātāpuna ki tai kia whakapakari ai te iwi Connecting and sustaining its people and communities for a positive future It is the Rangitīkei River that binds together the diverse hapū and iwi groups that occupy its banks OUR VALUES GUIDE OUR ACTIONS Tūpuna Awa | We are our Awa, our Awa is us Kōtahitanga | Working together with collective outcomes Kaitiakitanga | Maintaining and Enhancing the Mauri of the Awa and its tributaries Tino Rangatiratanga | Self Determination to develop and make our own decisions without impinging on the rights of others Manaakitanga | Duty of care to support other Hapū and Iwi where possible Mana Ātua | Recognising our spiritual association with Te Taiao Mana Tangata | Hapū and Iwi can exercise authority and control over Te Taiao through ahi kā and whakapapa Hau | Replenishing and enhancing a resource when it has been used Mana Whakahaere | Working Collaboratively for the Awa. 4 TE TAIAO The Awa, its trbutaries and ecosystems are revitalised and cared for by Hapū and Iwi, alongside the rest of the community through Focusing decision making on ensuring the mauri of the Awa is maintained and enhanced. -

The New Zealand Gazette 1703
Nov. 6] THE NEW ZEALAND GAZETTE 1703 Ruapuke, Public Hall. Remuera, Meadowbank Road, Presbyterian Church Hall. Rukuhia, Public Hall. Remuera, Rangitoto Avenue, Rawhiti Bowling Club Pavilion. Taupiri, Public Hall. Remuera, Remuera Road, North Memorial Baptist Church, Te Akau, Public Hall. Bible Class-room. Te Akau South, Ruku Ruku Public School. Remuera, Remuera Road No. 258, King's School. Te Hutewai, Public School. Remuera, Remuera Road, Public Library Lecture Hall Te Kohanga (Onewhero), Public Hall. (principal). Te Kowhai, Public Hall. Remuera, Remuera Road, Somervell Social Hall. Te Mata, Public Hall. Remuera, Upland Road No. llI, Mr. F. V. Anderson's Garage. Te Pahu, Public Hall. Remuera, Victoria Avenue, No. 136, Wilson Memorial Church. Te Rapa, Public Hall. Remuera, '\Vaiatarua Road, Meadowbank School. Te Uku, Memorial Hall. Waikaretu, Public School. Riccarton Electoral District- Waikokowai, Public Hall. Addington, Clarence Road and Leamington Street Corner, Waimai, Mr. H. Wilson's Residence. Marquee. Waingaro, Public Hall. Addington, Lincoln Road, Public Library. Wairamarama, Public School. Addington, Lincoln Road, Show-grounds. Waitetuna, Public School. Addington, Selwyn Street, Fancier's Hall. Whatawhata, Public Hall. Broadfields, Public School. Woodleigh, Matira Public Hall. Greenpark, Public School. Halswell, Public School. Rangitikei Electoral District Ladbrooks; Public School. Awahuri, Public Hall. Lincoln, Public Hall. Beaconsfield, Public School. Motukarara, Public Hall. Bonny Glen, Mr. L. A. Nitschke's Woolshed. Prebbleton, Public Library. Bulls, Town Hall. Riccarton, Centennial Avenue, St. Hilda's Mission HaJl. Cheltenham, Public School. Riccarton, Clarence Road, Town Hall (principal). Crofton, Mr. H. J. Calkin's Store. Riccarton, Matipo Street, Wharenui Public School. Cunninghams, Dunnolly, old Public School. Riccarton, Picton Avenue, No. -

ENVIRONMENTAL REPORT // 01.07.11 // 30.06.12 Matters Directly Withinterested Parties
ENVIRONMENTAL REPORT // 01.07.11 // 30.06.12 2 1 This report provides a summary of key environmental outcomes developed through the process to renew resource consents for the ongoing operation of the Tongariro Power Scheme. The process to renew resource consents was lengthy and complicated, with a vast amount of technical information collected. It is not the intention of this report to reproduce or replicate this information in any way, rather it summarises the key outcomes for the operating period 1 July 2011 to 30 June 2012. The report also provides a summary of key result areas. There are a number of technical reports, research programmes, environmental initiatives and agreements that have fed into this report. As stated above, it is not the intention of this report to reproduce or replicate this information, rather to provide a summary of it. Genesis Energy is happy to provide further details or technical reports or discuss matters directly with interested parties. HIGHLIGHTS 1 July 2011 to 30 June 2012 02 01 INTRODUCTION 02 1.1 Document Overview Rotoaira Tuna Wananga Genesis Energy was approached by 02 1.2 Resource Consents Process Overview members of Ngati Hikairo ki Tongariro during the reporting period 02 1.3 How to use this document with a proposal to the stranding of tuna (eels) at the Wairehu Drum 02 1.4 Genesis Energy’s Approach Screens at the outlet to Lake Otamangakau. A tuna wananga was to Environmental Management held at Otukou Marae in May 2012 to discuss the wider issues of tuna 02 1.4.1 Genesis Energy’s Values 03 1.4.2 Environmental Management System management and to develop skills in-house to undertake a monitoring 03 1.4.3 Resource Consents Management System and management programme (see Section 6.1.3 for details). -

TONGARIRO POWER SCHEME ENVIRONMENTAL REPORT // 01.07.12 30.06.13 ENVIRONMENTAL 13 Technical Reports Ordiscuss Matters Directly Withinterested Parties
TONGARIRO POWER SCHEME ENVIRONMENTAL REPORT // 01.07.12 30.06.13 ENVIRONMENTAL This report provides a summary of key environmental outcomes developed through the process to renew resource consents for the ongoing operation of the Tongariro Power Scheme. The process to renew resource consents was lengthy and complicated, with a vast amount of technical information collected. It is not the intention of this report to reproduce or replicate this information in any way, rather it summarises the key outcomes for the operating period 1 July 2012 to 30 June 2013 (referred to hereafter as ‘the reporting period’). The report also provides a summary of key result areas. There are a number of technical reports, research programmes, environmental initiatives and agreements that have fed into this report. As stated above, it is not the intention of this report to reproduce or replicate this information, rather to provide a summary of it. Genesis Energy is happy to provide further details or technical reports or discuss matters directly with interested parties. 13 HIGHLIGHTS 1 July 2012 to 30 June 2013 02 01 INTRODUCTION 02 1.1 Document Overview Te Maari Eruption Mount Tongariro erupted at the Te Maari Crater erupted on 02 1.2 Resource Consents Process Overview the 6 August and 21 November 2012. Both events posed a significant risk to 02 1.3 How to use this document the Tongariro Power Scheme (TPS) structures. During the August eruption, 02 1.4 Genesis Energy’s Approach which occurred at night, the Rangipo Power Station and Poutu Canal were to Environmental Management closed. -

Upper Ngaruroro River (Above Whanawhana)
Upper Ngaruroro River (above Whanawhana) Key Values Cultural Recreation (angling, rafting, kayaking) Ecology (wildlife, fisheries) Natural Character Landscape Table 1: List of documents reviewed Year Name Author 1966 An Encyclopaedia of New Zealand T.L Grant-Taylor 1979 64 New Zealand Rivers Egarr, Egarr & Mackay 1981 New Zealand Recreational River Survey G & J Egarr 1982 Submission of the draft Inventory of Wild and Scenic Rivers of National Ministry of Agriculture and Fisheries Importance 1984 The Relative Value of Hawke's Bay Rivers to New Zealand Anglers Fisheries Research Division - N.Z. Ministry of Agriculture and Fisheries 1986 A List of Rivers and Lakes Deserving Inclusion in A Schedule of Grindell & Guest Protected Waters 1988 Wildlife and Wildlife Habitat of Hawke’s Bay Rivers Department of Conservation 1994 Headwater Trout Fisheries in New Zealand NIWA 1994 Hawke’s Bay Conservancy – Conservation Management Strategy Department of Conservation 1998 Wildlife and Wildlife Habitat of Hawke’s Bay Rivers Department of Conservation 2004 Potential Water Bodies of National Importance Ministry for the Environment 2009 Angler Usage of Lake and River Fisheries Managed by Fish & Game Martin Unwin New Zealand: Results from the 2007/08 National Angling Survey- NIWA 2009 The 21 best fly fishing spots Stuff.co.nz 2010 Recreational Use of Hawke’s Bay Rivers – Results of the Recreational Hawke’s Bay Regional Council Usage Survey 2010 2011 Ngaruroro River Flood Protection and Drainage Scheme – Ecological MWH consultants Management and Enhancement -

Report on Aspects of the Wai 655 Claim / Waitangi Tribunal
R e p o r t o n A s p e c t s o f t h e W a i 6 5 5 C l a i m R e p o r t o n A s p e c t s o f t h e W a i 6 5 5 C l a i m W A I 6 5 5 W A I T A N G I T R I B U N A L R E P O R T 2 0 0 9 The cover design by Cliff Whiting invokes the signing of the Treaty of Waitangi and the consequent interwoven development of Māori and Pākehā history in New Zealand as it continuously unfolds in a pattern not yet completely known National Library of New Zealand Cataloguing-in-Publication Data New Zealand. Waitangi Tribunal. Report on aspects of the Wai 655 claim / Waitangi Tribunal. ISBN 978-1-86956-295-3 1. Treaty of Waitangi (1840) 2. Maori (New Zealand people)—Land tenure—New Zealand—Manawatu–Wanganui. 3. Maori (New Zealand people)—New Zealand—Manawatu–Wanganui—Claims. 4. Land tenure—New Zealand—Manawatu–Wanganui. [1. Tiriti o Waitangi. reo. 2. Mana whenua. reo] I. Title. 333.3309935—dc 22 www.waitangitribunal.govt.nz Typeset by the Waitangi Tribunal This report was previously released on the internet in 2009 in pre-publication format This amended edition published 2009 by Legislation Direct, Wellington, New Zealand Printed by SecuraCopy, Wellington, New Zealand 13 12 11 10 09 5 4 3 2 1 Set in Adobe Minion Pro and Cronos Pro Opticals Contents Chapter 1 : Introduction. -

Bibliography of Plant Checklists for Areas in Whanganui Conservancy
Bibliography of plant checklists for areas in Whanganui Conservancy MARCH 2010 Bibliography of plant checklists for areas in Whanganui Conservancy MARCH 2010 B Beale, V McGlynn and G La Cock, Whanganui Conservancy, Department of Conservation Published by: Department of Conservation Whanganui Conservancy Private Bag 3016 Wanganui New Zealand Bibliography of plant checklists for areas in Whanganui Conservancy - March 2010 1 Cover photo: Himatangi dunes © Copyright 2010, New Zealand Department of Conservation ISSN: 1178-8992 Te Tai Hauauru - Whanganui Conservancy Flora Series 2010/1 ISBN: 978-0-478-14754-4 2 Bibliography of plant checklists for areas in Whanganui Conservancy - March 2010 COntEnts Executive Summary 7 Introduction 8 Uses 10 Bibliography guidelines 11 Checklists 12 General 12 Egmont Ecological District 12 General 12 Mt Egmont/Taranaki 12 Coast 13 South Taranaki 13 Opunake 14 Ihaia 14 Rahotu 14 Okato 14 New Plymouth 15 Urenui/Waitara 17 Inglewood 17 Midhurst 18 Foxton Ecological District 18 General 18 Foxton 18 Tangimoana 19 Bulls 20 Whangaehu / Turakina 20 Wanganui Coast 20 Wanganui 21 Waitotara 21 Waverley 21 Patea 21 Manawatu Gorge Ecological District 22 General 22 Turitea 22 Kahuterawa 22 Manawatu Plains Ecological District 22 General 22 Hawera 23 Waverley 23 Nukumaru 23 Maxwell 23 Kai Iwi 23 Whanganui 24 Turakina 25 Bibliography of plant checklists for areas in Whanganui Conservancy - March 2010 3 Tutaenui 25 Rata 25 Rewa 25 Marton 25 Dunolly 26 Halcombe 26 Kimbolton 26 Bulls 26 Feilding 26 Rongotea 27 Ashhurst 27 Palmerston