2013AB Release from 11/21/2013
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

University of Washington Special Collections
UNIVERSITY CHRONOLOGY 1850 to 1859 February 28, 1854 Governor Isaac Ingalls Stevens recommended to the first territorial legislature a memorial to Congress for the grant of two townships of land for the endowment for a university. (“That every youth, however limited his opportunities, find his place in the school, the college, the university, if God has given him the necessary gifts.” Governor Stevens) March 22, 1854 Memorial to Congress passed by the legislature. January 29, 1855 Legislature established two universities, one in Lewis County and one in Seattle. January 30, 1858 Legislature repealed act of 1855 and located one university at Cowlitz Farm Prairies, Lewis County, provided one hundred and sixty acres be locally donated for a campus. (The condition was never met.) 1860 to 1869 December 12, 1860 Legislature passed bill relocating the university at Seattle on condition ten acres be donated for a suitable campus. January 21, 1861 Legislative act was passed providing for the selection and location of endowment lands reserved for university purposes, and for the appointment of commissioners for the selection of a site for the territorial university. February 22, 1861 Commissioners first met. “Father” Daniel Bagley was chosen president of the board April 16, 1861 Arthur A. Denny, Edward Lander, and Charles C. Terry deeded the necessary ten acres for the campus. (This campus was occupied be the University until 1894.) May 21, 1861 Corner stone of first territorial University building was laid. “The finest educational structure in Pacific Northwest.” November 4, 1861 The University opened, with Asa Shinn Mercer as temporary head. Accommodations: one room and thirty students. -

VII. STANDING COMMITTEES B. Finance and Asset Management
F–5 VII. STANDING COMMITTEES B. Finance and Asset Management Committee Denny Hall Renovation – Approve Revised Budget and Finance Plan J F M A M J J A S O N D J F M A M J J A S O N D J F M A M J J A S O N D J F M A M J J A S O N D INFORMATION 2013 2014 2015 2016 PHASES 2013 2014 2015 2016 ACTION J F M A M J J A S O N D J F M A M J J A S O N D J F M A M J J A S O N D J F M A M J J A S O N D May 2007 January 2008 January 2015 Select Architect Adopt Budget Approve Revised Approve Use of Budget and Approve Alternative Public Debt Funding Works Delegate Award of GC/CM Contract Note for duration of project: Semi-annual reports in January & July RECOMMENDED ACTIONS It is the recommendation of the administration and the Finance and Asset Management Committee that the Board of Regents: 1. Approve a decrease to the Denny Hall project budget from $56.9 million to $52.9 million; and 2. Approve the debt funding financing plan, including the use of up to $16.16 million of general revenue bond proceeds toward the project. PROJECT BACKGROUND Constructed in 1895, Denny Hall is the oldest building on the Seattle campus and is listed in the Washington State Heritage Register. Denny Hall has been prioritized as part of the University’s ongoing “Restoring the Core” renovation program to restore and modernize buildings in greatest need of renovation. -

Montlake Walk III: University of Washington and Ravenna Park
Montlake Walk III: University of Washington and Ravenna Park The University of Washington campus was the site of Seattle’s first World’s Fair in 1909. John Olmsted of Olmsted Brothers designed the fairgrounds for the ALASKA- YUKON-PACIFIC EXPOSITION. Although most of the buildings were temporary, some of them have remained on campus since 1909. Turn left from Fuel down Lynn, and take another left on 25th. Continue until 25th ends, turn left and then right to arrive at Lake Washington Boulevard. Turn left and walk to Montlake Boulevard. Cross Lake Washington Boulevard at the light. Walk across the bridge over Highway 520 and the Montlake Bridge. Continue to walk to the new bike/pedestrian bridge over Montlake Boulevard. Walk on the bridge until you arrive at Rainier Vista, designed by the Olmsteds as part of the A-Y-P Exposition. Continue to Drumheller Fountain, and walk up a set of stairs to Red Square. Suzzallo Library is located on the right. Restrooms are located here. Exit and angle right between Kane Hall and the library until you find yourself in the Liberal Arts Quadrangle (The Quad). Exit the Quad on the left, just before Raitt Hall. The building in front of you is Denny Hall. It was built in 1895 and is the oldest building on campus. Turn left and angle around Denny Hall until you reach Memorial Way. It is lined with 58 sycamore trees to represent the 58 UW students and faculty who lost their lives in World War I. Turn right on Memorial Way. Cross NE 45th St., and walk along 17th Avenue. -

Hutchinson Hall UW Historic Resources Addendum
Hutchinson Hall UW Historic Resources Addendum BOLA Architecture + Planning June 8, 2012 1. INTRODUCTION Background The University of Washington is proposing to reroof portions of Hutchinson Hall, which was built in 1927 as the Women’s Physical Education Building. The building is located in the north area of campus, which dates from its establishment in the late 19th century. The proposed project will involve repairs or replacement of deteriorated, original and non-original roofing, which consists of slate and composition shingles, as well as membrane materials at built-in gutters and flat roof areas. The original roof also has copper flashing, ridge cresting, eave gutters and downspouts. Consistent with its historic preservation policies, as outlined in its “University of Washington Master Plan—Seattle Campus” of January 2003 (2003 Seattle Campus Master Plan), the University of Washington sought historic and urban design information about Hutchinson Hall in a Historic Resources Addendum (HRA). This type of report is developed for any project that makes exterior alterations to a building over 50 years old, or is adjacent to a building or a significant campus feature older than 50 years. Hutchinson Hall is subject to this requirement because of its age. An HRA is required also for public spaces identified in Fig. III-2 of the 2003 Seattle Campus Master Plan. This report provides historical and architectural information about the building, a preliminary evaluation of its historic significance to the University, information about the proposed project, and recommendations. A bibliography and list of source documents is provided at the end of the text, followed by original drawings, building plans, and historic and contemporary photographs. -

University of Washington Minimax Program
UNIVERSITY OF WASHINGTON MINIMAX PROGRAM FEBRUARY 26, 2019 THE UNIVERSITY OF WASHINGTON ORGANIZATION CHART Custodial Services Building UW Facilities 255 staff Services Dept. 990 staff 285 staff UW Recycling : Key campus partners include 12 staff • Housing & Food Services • University of Washington Medical Center • Intercollegiate Athletics • UW Sustainability WHAT IS MINIMAX? MINIMIZE WASTE, MAXIMIZE RECYCLING Custodial-serviced Self-serviced Standalone garbage Big Recycling & Small Waste Lined Unlined Mismatched Streamlined WHAT IS MINIMAX? MINIMIZE WASTE, MAXIMIZE RECYCLING Garbage-centric Diversion-centric Paper towel Garbage-only composting & small restrooms waste UW WASTE Waste 100% 90% Waste Sent to Landfill 80% 2020 Target: 70% 70% 60% 50% 40% 30% 20% Waste Diverted from Landfill 10% (composted, recycled, resold) 0% BUILD THE CASE UW WASTE COLLECTION 2005: Ordinance 2010: MiniMax 2013: 2015: bans paper 2008: standard in new Restroom Ordinance from landfill MiniMax Pilot and renovated paper towel bans food from buildings compost pilot landfill 2004: Pre- 2006: 2009: Extended 2011: 2013: UW 2018: consumer food Voluntary MiniMax Piloted Garbology MiniMax waste & coffee compost to voluntary public area Project implemented grounds program buildings compost 100% collected UW GARBOLOGY PROJECT QUANTIFYING THE IMPACT UW anthropology students held trash audits before / after MiniMax at Denny Hall: > Increased Denny Hall’s waste diversion by 10% (42.6% to 52.5%) > 83 tons diverted annually > 3.5% reduction in disposal cost INSTITUTIONALIZED -
Uwtacoma-Phase 2B
C–6 VII. STANDING COMMITTEE C. Capital Assets Committee ACTIONS TAKEN UNDER DELEGATED AUTHORITY Reported to the Capital Assets Committee on January 20, 2005 Pursuant to the Standing Orders of the Board of Regents, Delegation of Authority, and to the delegation of authority from the President of the University to the Executive Vice President in Executive Order No. 1, to take action for projects or contracts that exceed $1,000,000 in value or cost but are less than $5,000,000, the Administration may approve and execute all instruments. Project Name: Kane Hall Classroom Improvements, Project No.: 10720 Action Reported: Architect Appointment and Establish Project Budget On September 15, 2004 an agreement for architectural services was awarded to Hoshide Williams Architects (HWA) for the Kane Hall Classroom Improvements project, as an authorization under their term agreement. HWA was founded in 1984 and currently has authorizations under their master agreement to design projects on the Seattle and Friday Harbor campuses, including classroom renovations in Gowen, Communications and Hitchcock Halls. HWA is currently working on the Seattle Public Library’s Fremont Branch Library. This project will provide new finishes, new seating, ceiling replacement, acoustical improvements, and new audio visual equipment to three classrooms in Kane Hall – rooms 120, 210, and 220. The project is approximately 17,700 gross square feet and is anticipated to be completed during summer quarter 2005 for occupancy at the start of fall quarter. The project budget is $3,541,520. Funding is from the 2004 Supplemental Capital Budget.. Budget Summary: Original Apprv’d Revised Budget Forecast/Actual Budget (Date) 10/4/04 Total Consultant Svcs $420,175 $420,175 Total Construction Cost $2,549,232 $2,549,232 Other Costs $341,208 $341,208 Project Administration $230,905 $230,905 Total Project Budget $3,541,520 $3,541,520 1 Denny Hall Roof Replacement, Project No. -

Husky Baseball Facilities 2007 SEASON Husky Ballpark Washington, Which Had Played at Graves Field Since the 1960S, Opened Husky Ballpark in 1998
THE HUSKY EXPERIENCE Husky Baseball Facilities 2007 SEASON HUSKY BALLPARK Washington, which had played at Graves Field since the 1960s, opened Husky Ballpark in 1998. In eight years, the UW has already played 225 home games – more than 28 per season. Additionally, hundreds of games involving youth, semi-pro and recreational teams are played at Husky Ballpark each summer. The Huskies will continue to play at the new park, PLAYERS consistenly improving the existing temporary setting in anticipation of a new stadium being constructed in the future. Even without the stadium completed, Husky Ballpark is a step up for the program. Here’s a rundown of what’s been completed: The Infield The infield consists of a new (installed in 2005), state- STAFF of-the-art FieldTurf surface. The new turf replaced the original AstroTurf last season and, while improving the quality of play, has also greatly increased the asthetic look of the park with the more natural-looking material. Husky Ballpark sits on the eastern edge of the UW campus on the shores of Union Bay and Lake Washington The Outfield The outfield is a natural grass surface with a complete in the first base dugout and the visitors at third base. Both 2006 REVIEW in-ground irrigation and state of the art drainage system. are equipped with a storage area at the outfield end and The grass is a mixture of bluegrass, rye and fescue, which both dugouts will be replaced with new dugouts when will make the facility playable in any weather. the stadium is built. The Warning Track The Bullpens The entire field is surrounded by a 12-foot wide synthetic The home bullpen is located in foul territory on the right warning track. -
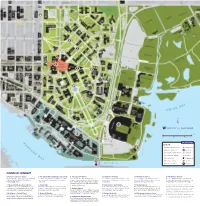
1 13 14 12 11 Points of Interest 15
5 6 4 3 1 2 7 8 9 10 13 12 0 ¼ mile 11 0 500 1,000 feet uw.edu/maps LEGEND 14 Museums & galleries Information Points of interest Gatehouse Lecture & performance Light rail Husky merchandise Bus stop Ticket office Parking gate 15 Campus dining UW Police Cafés & markets Hospital POINTS OF INTEREST Information and Visitor Center 3 The Liberal Arts Quadrangle (the Quad) 6 University Book Store 9 Drumheller Fountain 12 UW Medical Center 15 UW Botanic Gardens The Visitor Center is next to the George Washington The Quad is the primary gathering place on campus, Located on the Ave, University Book Store is a The centerpiece of Rainier Vista, Drumheller Foun- One of the highest-ranked medical centers in Across the Montlake Bridge are the UW Botanic statue on the ground floor of the Odegaard especially when the Yoshino cherry trees bloom thriving independent bookstore that regularly hosts tain was built to highlight our spectacular view of America, UW Medical Center is also home to the Gardens and Washington Park Arboretum, one of Undergraduate Library. each spring. author events, signings and book clubs, in addition Mount Rainier. top-ranked UW School of Medicine. the oldest arboretums west of the Mississippi. to being the UW source for textbooks, art supplies, 1 Odegaard Undergraduate Library 4 Denny Hall technology and Husky gear. 10 Sylvan Grove and Columns 13 Husky Stadium The University of Washington is committed to In addition to offering research and writing services, Built in 1895, Denny Hall is the oldest building on At the south end of this picturesque shady grove With views of Lake Washington and the Cascade providing access, equal opportunity and reasonable Odegaard is home to By George Café. -

The University of Washington
THE UNIVERSITY OF WASHINGTON Founded in 1861, the University of Washington is one of the foremost institutions of higher education in the nation, richly combining its research, instruc- tional and public service missions. Its internationally acclaimed faculty includes five Nobel laureates and the winner of the 1990 National Book Award for fiction. Washington is part of an elite group of research universities whose contributions to American life are unique because they generate the basic knowledge upon which practical innovations are based. The UW student body on the Seattle campus totals about 37,000, with an undergraduate enrollment of approximately 26,800. The UW also has campuses in Bothell and Tacoma, designed primarily for upper division (junior and senior) undergraduates and master’s level graduate programs. Total enrollment at these campuses is about 3,600. For more than 30 years, the university has been among the country’s top five institutions in the dollar value of federal research grants and contracts awarded to its faculty. In 2001, the most recent year for which that data has been collected, the UW ranked second overall and first among public universities. Total grant and contract activity for 2002 exceeded $800 million. More than 80 percent of the university’s grant and contract funds come from federal agencies. Research contrib- utes directly to the educational goals of graduate and professional students, as well as to those of undergraduates. Instruction and research at Washington are supported by a library system that is one of the most extensive in the nation, consisting of five major units and 18 branches, as well as libraries at UW Tacoma and UW Bothell, together housing more than five million volumes. -

Parrington Hall Nomination
Parrington Hall UNIVERSITY OF WASHINGTON NORTHWEST VERNACULAR May 2018 Parrington Hall Landmark Nomination University of Washington Page 1 Table of Contents Landmarks Application Page 2 1. Property Data 3 2. Architectural Description 4 Campus Setting and Site 4 The Building and Changes through Time 4 3. Historic Context and Significance 17 Early Development of the University 17 Construction and Use of the Building 19 Architects 20 4. Endnotes 26 5. Bibliography 28 6. List of Figures 30 NORTHWEST VERNACULAR Landmarks Application Page Name: Parrington Hall (Science Hall) Year Built: 1902 Street and Number: 4105 Memorial Drive NE (parcel address: 4000 15th Avenue NE) Assessor’s File No: 1625049001 (portion of) Legal Description: Those portions of Government Lots 2, 3 and 4, lying west of Montlake Blvd NE, north of NE Pacific Street and north of NE Pacific Place; the west ½ of the northwest ¼, and the northwest ¼ of the southwest ¼, lying east of 15th Avenue NE and south of NE 45th Street and north of NE Pacific Street; all in Section 16, T25N, R4E, W.M. Plat Name: Block: Lot: Present Owner: University of Washington Present Use: Classrooms, Offices, Evans Graduate School of Public Policy Address: Julie Blakeslee, Environmental and Land Use Planner Capital Planning & Development University Facilities Building, Box 352205 Seattle, WA 98195‐220505 Original Owner: University of Washington Original Use: Classrooms, Offices (Science departments) Architect: Josenhans & Allan Submitted by: Katie Pratt and Spencer Howard, Northwest Vernacular on behalf of Julie Blakeslee, UW Capital Planning & Development Address: 3377 Bethel Rd SE, Suite 107 #318, Port Orchard, WA 98366 Phone: (360) 813-0772 Date: Reviewed: Date: (Historic Preservation Officer) Parrington Hall Landmark Nomination University of Washington Page 3 1. -

UNIVERSITY VILLAGE to UNIVERSITY of WASHINGTON – 5.1 Miles
UNIVERSITY VILLAGE TO UNIVERSITY OF WASHINGTON – 5.1 miles Start at University Village Starbucks, 4634 26th Ave. NE and walk to the west entrance of University Village. Cross 25th Ave. NE at the light, and walk north until you reach the Burke- Gilman Trail. Cross NE Blakeley St. and angle left to continue walking along Ravenna Place NE. Cross NE 55th St. to RAVENNA PARK. Walk north on the path that parallels Ravenna Ave. NE. Turn left at the small parking lot at the west edge of the park. Walk west on NE Ravenna Blvd. until you come to 20th Ave. NE.. Cross Ravenna and turn right. Continue walking until you reach 17th Ave. NE. Turn left and walk to NE 45th St. The Olmsted Brothers designed both NE Ravenna Blvd. and 17th Ave. NE. The houses in the University Park area were built in the early 20th century. One of the first houses (built in 1909) is on the southwest corner of 17th Ave. NE and NE 55th St. Cross NE 45th St. at the light and enter the University of Washington campus. John Olmsted of Olmsted Brothers designed the fairgrounds for the ALASKA-YUKON-PACIFIC EXPOSITION (A-Y-P-E). It was Seattle’s first World’s Fair on the UW campus in 1909. Follow Memorial Way past Stevens Lane. Just past Clallam Place is DENNY HALL on the left. It was built in 1895 and is the oldest building on the university campus. George Washington Lane angles right at the War Memorial. The building on your right is PARRINGTON HALL. -

University of Washington 2011-2013 Capital Budget Request and 2011-2021 Capital Plan September 2010
University of Washington 2011‐2013 Capital Budget Request and 2011‐2021 Capital Plan September 2010 University of Washington 2011-13 Capital Budget Request September 1, 2010 2011‐2013 Capital Budget Request and 2011‐2021 Capital Plan September 2010 TABLE OF CONTENTS TAB A –STRATEGIC FRAMEWORK 1 University of Washington Strategic Framework 3 University of Washington 2011‐2013 Capital Request ‐ By Fund 8 2011 ‐ 2021 Ten Year Capital Plan Summary (UW Format) 9 2011‐2021 Ten Year Capital Plan Summary (OFM Format) 10 Ten Year Capital Plan by Priority 10 Ten Year Capital Plan by Project Class 15 Department of Archaeology and Historic Preservation Review 21 Capital FTE Summary 25 TAB B ‐ PRESERVATION PROJECTS 29 Preservation Projects Capital Project Request Summary 31 Capital Project Requests (in priority order within category) 41 Minor Capital Repair ‐ Infrastructure, Health, Safety 41 UW High Voltage Improvement Project 89 Denny Hall Renovation 106 Anderson Hall Renovation 116 Lewis Hall Renovation 126 Miller Hall Renovation 136 UW Seattle Major Infrastructure Projects 146 UW Tacoma Major Infrastructure Projects 151 UW Bothell Major Infrastructure Projects 160 Hutchinson Hall Renovation 164 College of Engineering Academic Building ‐ Renovation 174 College of Arts & Sciences Building ‐ Renovation 184 Eagleson Hall Renovation 195 Minor Works‐ Facility Preservation 205 Safe Campus 208 UW Tacoma Soils Remediation 211 Infrastructure Savings 213 2011‐2013 Capital Budget Request and 2011‐2021 Capital Plan September 2010 TABLE OF CONTENTS (continued)