Possible Spillover of Pathogens Between Bee Communities Foraging on the Same Floral Resource
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Global Trends in Bumble Bee Health
EN65CH11_Cameron ARjats.cls December 18, 2019 20:52 Annual Review of Entomology Global Trends in Bumble Bee Health Sydney A. Cameron1,∗ and Ben M. Sadd2 1Department of Entomology, University of Illinois, Urbana, Illinois 61801, USA; email: [email protected] 2School of Biological Sciences, Illinois State University, Normal, Illinois 61790, USA; email: [email protected] Annu. Rev. Entomol. 2020. 65:209–32 Keywords First published as a Review in Advance on Bombus, pollinator, status, decline, conservation, neonicotinoids, pathogens October 14, 2019 The Annual Review of Entomology is online at Abstract ento.annualreviews.org Bumble bees (Bombus) are unusually important pollinators, with approx- https://doi.org/10.1146/annurev-ento-011118- imately 260 wild species native to all biogeographic regions except sub- 111847 Saharan Africa, Australia, and New Zealand. As they are vitally important in Copyright © 2020 by Annual Reviews. natural ecosystems and to agricultural food production globally, the increase Annu. Rev. Entomol. 2020.65:209-232. Downloaded from www.annualreviews.org All rights reserved in reports of declining distribution and abundance over the past decade ∗ Corresponding author has led to an explosion of interest in bumble bee population decline. We Access provided by University of Illinois - Urbana Champaign on 02/11/20. For personal use only. summarize data on the threat status of wild bumble bee species across bio- geographic regions, underscoring regions lacking assessment data. Focusing on data-rich studies, we also synthesize recent research on potential causes of population declines. There is evidence that habitat loss, changing climate, pathogen transmission, invasion of nonnative species, and pesticides, oper- ating individually and in combination, negatively impact bumble bee health, and that effects may depend on species and locality. -

Bumble Bees in Montana
Bumble Bees in Montana by Amelia C. Dolan, Middle School Specialist at Athlos Academies, Boise, ID and former MSU Graduate Student; Casey M. Delphia, Research Scientist, Departments of Ecology and LRES; and Lauren M. Kerzicnik, Insect Diagnostician and Assistant IPM Specialist Bumble bees are important native pollinators in wildlands and agricultural systems. Creating habitat to support bumble bees in yards and gardens can MontGuide be easy and is a great way to get involved in native bee conservation. MT201611AG New 7/16 BUMBLE BEES ARE IMPORTANT NATIVE Bumble bees are one group of bees that are able to pollinators in wildlands and agricultural systems. They “buzz pollinate,” which is important for certain types are easily recognized by their large size and colorful, hairy of plants such as blueberries and tomatoes. Within bodies. Queens are active in the spring and workers can the flowers of these types of plants, pollen is held in be seen throughout the summer into early fall. Creating small tube-like anthers (i.e. poricidal anthers), and is habitat to support bumble bees in yards and gardens can not released unless the anthers are vibrated. Bumble be easy and is a great way to get involved in native bee bees buzz pollinate by landing on the flower, grabbing conservation. the anthers with their jaws (i.e. mandibles), and then Bumble bees are in the family Apidae (includes honey quickly vibrating their flight muscles. The vibration bees, bumble bees, carpenter bees, cuckoo bees, sunflower effect is similar to an electric toothbrush and the pollen bees, and digger bees) and the is released. -

The Conservation Status of Bumble Bees of Canada, the USA, and Mexico
The Conservation Status of Bumble Bees of Canada, the USA, and Mexico Scott Hoffman Black, Rich Hatfield, Sarina Jepsen, Sheila Colla and Rémy Vandame Photo: Clay Bolt Importance of Bumble Bees There are 57 bumble bee species in Canada, US and Mexico. Hunt’s bumble bee; Bombus huntii , Photo: Clay Bolt Importance of Bumble Bees Important pollinator of many crops. Bombus sandersoni , Sanderson’s bumble bee Photo: Clay Bolt Importance of Bumble Bees Keystone pollinator in ecosystems. Yelllowheaded bumble bee, Bombus flavifrons Photo: Clay Bolt Bumble Bee Extinction Risk Assessment Network of 75+ bumble bee experts & specialists worldwide Goal: global bumble bee extinction risk assessment using consistent criteria Bumble Bee Extinction Risk Assessment IUCN Red List Criteria for Evaluating Extinction Risk • Used a database of 250,000+ specimen records (created from multiple data providers and compiled by Leif Richardson for Bumble Bees of North America) • Evaluate changes between recent (2002-2012) and historic (pre-2002): • Range (Extent of Occurrence) • Relative abundance • Persistence (50 km x 50 km grid cell occupancy) Sources: Hatfield et al. 2015 Bumble Bee Extinction Risk Assessment IUCN Red List Criteria for Evaluating Extinction Risk • Analyses informed application of Red List Categories; BBSG members provided review • This method was then adapted applied to South American and Mesoamerican bumble bees. Sources: Hatfield et al. 2015 Bumble Bee Extinction Risk Assessment Trilateral Region 6 4 5 28% of bumble bees Critically Endangered in Canada, the Endangered United States, and 7 Vulnerable Mexico are in an IUCN Threatened Near Threatened Category Least Concern 3 Data Deficient 32 Sources: Hatfield et al. -

Bumble Bee Surveys in the Columbia River Gorge National Scenic Area of Oregon and Washington
Bumble Bee Surveys in the Columbia River Gorge National Scenic Area of Oregon and Washington Final report from the Xerces Society to the U.S. Forest Service and Interagency Special Status/Sensitive Species Program (ISSSSP) Agreement L13AC00102, Modification 5 Bombus vosnesenskii on Balsamorhiza sagittata. Photo by Rich Hatfield, the Xerces Society. By Rich Hatfield, Sarina Jepsen, and Scott Black, the Xerces Society for Invertebrate Conservation September 2017 1 Table of Contents Abstract ......................................................................................................................................................... 3 Introduction .................................................................................................................................................. 3 Methods ........................................................................................................................................................ 6 Site Selection ............................................................................................................................................. 6 Site Descriptions (west to east) ................................................................................................................ 7 T14ES27 (USFS) ..................................................................................................................................... 7 Cape Horn (USFS) ................................................................................................................................. -

Comparative Behavioral Biology of Two Middle East Species of Carpenter Bees (Xylocopa Latreille) (Hymenoptera: Apoidea)
Comparative Behavioral Biology of Two Middle East Species of Carpenter Bees (Xylocopa Latreille) (Hymenoptera: Apoidea) DAN GERLING, PAUL D. HURD, JR., and ABRAHAM HEFETZ SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 369 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoo/ogy Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world of science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

Rna Virus Ecology in Bumble Bees (Bombus Spp.) and Evidence for Disease Spillover Samantha Ann Alger University of Vermont
University of Vermont ScholarWorks @ UVM Graduate College Dissertations and Theses Dissertations and Theses 2018 Rna Virus Ecology In Bumble Bees (bombus Spp.) And Evidence For Disease Spillover Samantha Ann Alger University of Vermont Follow this and additional works at: https://scholarworks.uvm.edu/graddis Part of the Ecology and Evolutionary Biology Commons Recommended Citation Alger, Samantha Ann, "Rna Virus Ecology In Bumble Bees (bombus Spp.) And Evidence For Disease Spillover" (2018). Graduate College Dissertations and Theses. 955. https://scholarworks.uvm.edu/graddis/955 This Dissertation is brought to you for free and open access by the Dissertations and Theses at ScholarWorks @ UVM. It has been accepted for inclusion in Graduate College Dissertations and Theses by an authorized administrator of ScholarWorks @ UVM. For more information, please contact [email protected]. RNA VIRUS ECOLOGY IN BUMBLE BEES (BOMBUS SPP.) AND EVIDENCE FOR DISEASE SPILLOVER A Dissertation Presented by Samantha A. Alger to The Faculty of the Graduate College of The University of Vermont In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Specializing in Biology October, 2018 Defense Date: August 31, 2018 Dissertation Examination Committee: Alison K. Brody, Ph.D., Advisor Taylor Ricketts Ph.D., Chairperson Joseph Schall, Ph.D. Sara Helms Cahan, Ph.D. Brian Voigt, Ph.D. Cynthia J. Forehand, Ph.D., Dean of the Graduate College ABSTRACT The inadvertent spread of exotic pests and pathogens has resulted in devastating losses for bees. The vast majority of bee disease research has focused on a single species of managed bee, the European honey bee ( Apis mellifera ). -
(Hymenoptera, Apoidea, Anthophila) in Serbia
ZooKeys 1053: 43–105 (2021) A peer-reviewed open-access journal doi: 10.3897/zookeys.1053.67288 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research Contribution to the knowledge of the bee fauna (Hymenoptera, Apoidea, Anthophila) in Serbia Sonja Mudri-Stojnić1, Andrijana Andrić2, Zlata Markov-Ristić1, Aleksandar Đukić3, Ante Vujić1 1 University of Novi Sad, Faculty of Sciences, Department of Biology and Ecology, Trg Dositeja Obradovića 2, 21000 Novi Sad, Serbia 2 University of Novi Sad, BioSense Institute, Dr Zorana Đinđića 1, 21000 Novi Sad, Serbia 3 Scientific Research Society of Biology and Ecology Students “Josif Pančić”, Trg Dositeja Obradovića 2, 21000 Novi Sad, Serbia Corresponding author: Sonja Mudri-Stojnić ([email protected]) Academic editor: Thorleif Dörfel | Received 13 April 2021 | Accepted 1 June 2021 | Published 2 August 2021 http://zoobank.org/88717A86-19ED-4E8A-8F1E-9BF0EE60959B Citation: Mudri-Stojnić S, Andrić A, Markov-Ristić Z, Đukić A, Vujić A (2021) Contribution to the knowledge of the bee fauna (Hymenoptera, Apoidea, Anthophila) in Serbia. ZooKeys 1053: 43–105. https://doi.org/10.3897/zookeys.1053.67288 Abstract The current work represents summarised data on the bee fauna in Serbia from previous publications, collections, and field data in the period from 1890 to 2020. A total of 706 species from all six of the globally widespread bee families is recorded; of the total number of recorded species, 314 have been con- firmed by determination, while 392 species are from published data. Fourteen species, collected in the last three years, are the first published records of these taxa from Serbia:Andrena barbareae (Panzer, 1805), A. -
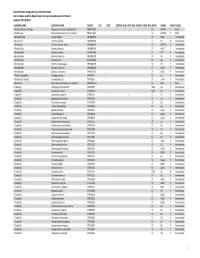
Element Status Designations by Common Name Arizona Game And
Element Status Designations by Common Name Arizona Game and Fish Department, Heritage Data Management System Updated: 10/15/2019 COMMON NAME SCIENTIFIC NAME ELCODE ESA DATE CRITHAB BLM USFS NESL MEXFED SGCN NPL SRANK GRANK TRACK TAXON A Arizona‐Mexican Orange Choisya arizonica var. amplophylla PDRUT02031 S2 G4TNR Y Plant A Balsamroot Balsamorhiza hookeri var. hispidula PDAST11041 S1 G5T3T5 Y Plant A Blueberry Bee Osmia ribifloris IIHYMA2570 S? G4G5 Y Invertebrate A Buckmoth Hemileuca grotei IILEW0M070 S? G4 N Invertebrate A Buckmoth Hemileuca grotei diana IILEW0M072 S? G4T3T4 Y Invertebrate A Bumble Bee Bombus centralis IIHYM24100 S? G4G5 Y Invertebrate A Bumble Bee Bombus fervidus IIHYM24110 S? G4? Y Invertebrate A Bumble Bee Bombus flavifrons IIHYM24120 S? G5 Y Invertebrate A Bumble Bee Bombus huntii IIHYM24140 S? G5 Y Invertebrate A Bumble Bee Bombus melanopygus IIHYM24150 S? G5 Y Invertebrate A Bumble Bee Bombus morrisoni IIHYM24460 S? G4G5 Y Invertebrate A Bumble Bee Bombus nevadensis IIHYM24170 S? G4G5 Y Invertebrate A Bushtail Caddisfly Gumaga griseola IITRI53010 S? G5 Y Invertebrate A Bushtailed Caddisfly Gumaga nigricula IITRI53020 S? G3G4 Y Invertebrate A Buttercup Ranunculus inamoenus var. subaffinis PDRAN0L1C3 S1 G5T1 YPlant A caddisfly Hydropsyche occidentalis IITRI25460 S2S3 G5 Y Invertebrate A caddisfly Hydropsyche oslari IITRIG6010 S2S3 G5 Y Invertebrate A caddisfly Lepidostoma apache IITRI64A10 S S1 G1 Y Invertebrate A Caddisfly Agapetus boulderensis IITRI33190 S? G5 Y Invertebrate A Caddisfly Alisotrichia arizonica IITRID7010 -

Journal of Melittology Bee Biology, Ecology, Evolution, & Systematics the Latest Buzz in Bee Biology No
Journal of Melittology Bee Biology, Ecology, Evolution, & Systematics The latest buzz in bee biology No. 38, pp. 1–9 26 August 2014 The effect of photobleaching on bee (Hymenoptera: Apoidea) setae color and its implications for studying aging and behavior Jonathan B. Koch1, Byron Love1,2, Ellen Klinger1,2, & James P. Strange2 Abstract. Historically, bee age has been estimated using measurements of wing wear and in- tegument color change. These measurements have been useful in studies of foraging ecology and plant-pollinator interactions. Wing wear is speculated to be affected by the behaviors as- sociated with foraging, nesting, and mating activities. Setal color change may be an additional parameter used to measure bee age if it is affected by sun exposure during these same activities. The objectives of this study were to experimentally assess the effect of direct sun exposure on setal color, unicellular hair-like processes of the integument, and determine whether wing wear and integument photobleaching are correlated. To quantify photobleaching of setae, we mea- sured changes in hue of lab-reared Bombus huntii Greene (Apidae) exposed to natural sunlight. We found that sun exposure was a significant variable in determining setal bleaching. To assess the relationship between wing wear and setal photobleaching, we scored wing wear and mea- sured setal hue of B. huntii, Melecta pacifica fulvida Cresson (Apidae), and Osmia integra Cresson (Megachilidae) from museum specimens. Wing wear and setal hue values were positively cor- related for all three species; however, the strength of the relationship varies across bee species as indicated by correlation coefficient estimates. -

Bumble Bees (Hymenoptera: Apidae) of Montana (PDF)
Bumble Bees (Hymenoptera: Apidae) of Montana Authors: Amelia C. Dolan, Casey M. Delphia, Kevin M. O'Neill, and Michael A. Ivie This is a pre-copyedited, author-produced PDF of an article accepted for publication in Annals of the Entomological Society of America following peer review. The version of record for (see citation below) is available online at: https://dx.doi.org/10.1093/aesa/saw064. Dolan, Amelia C., Casey M Delphia, Kevin M. O'Neill, and Michael A. Ivie. "Bumble Bees (Hymenoptera: Apidae) of Montana." Annals of the Entomological Society of America 110, no. 2 (September 2017): 129-144. DOI: 10.1093/aesa/saw064. Made available through Montana State University’s ScholarWorks scholarworks.montana.edu Bumble Bees (Hymenoptera: Apidae) of Montana Amelia C. Dolan,1 Casey M. Delphia,1,2,3 Kevin M. O’Neill,1,2 and Michael A. Ivie1,4 1Montana Entomology Collection, Montana State University, Marsh Labs, Room 50, 1911 West Lincoln St., Bozeman, MT 59717 ([email protected]; [email protected]; [email protected]; [email protected]), 2Department of Land Resources and Environmental Sciences, Montana State University, Bozeman, MT 59717, 3Department of Ecology, Montana State University, Bozeman, MT 59717, and 4Corresponding author, e-mail: [email protected] Subject Editor: Allen Szalanski Received 10 May 2016; Editorial decision 12 August 2016 Abstract Montana supports a diverse assemblage of bumble bees (Bombus Latreille) due to its size, landscape diversity, and location at the junction of known geographic ranges of North American species. We compiled the first in- ventory of Bombus species in Montana, using records from 25 natural history collections and labs engaged in bee research, collected over the past 125 years, as well as specimens collected specifically for this project dur- ing the summer of 2015. -

Pollinator Management Guide
Chase M. Wasicuna Land and Water Management Assiniboine Community College, City of Brandon April 3, 2017 Acknowledgments This Management Guide was designed in partnership with and assistance from: - The City of Brandon - Lindsay Hargreaves, City of Brandon - Sherry Punak-Murphy, CFB Shilo - Melanie Dubois, Agriculture and Agri-food Canada, Manitoba - Mark Wonneck, Agriculture and Agri-food Canada, Alberta - Neal Hackler, Assiniboine Community College I would like to thank everyone who helped provide information and input for the development of this guide. Annotated Bibliography The decline of bees has begun to gain recognition; however, the general public does not recognise the level of decline in native pollinators. “There hasn't been a lot of attention paid to native bees, which include well-known bumblebees and lesser-known varieties such as leafcutting bees or orchard mason bees.” Dyson (2016). Raising awareness of the local native species is a key part to developing an inclusive pollinator program. Not all pollinators are easily recognizable like the many species of bumblebees, but even if you don’t see them they are still an important part of the food production “…since bees pollinate 75 percent of fruits, nuts, and vegetables grown in North America.” Weidenhammer (2016). In order to maintain a healthy pollinator population in any location you must have the resources available for them. Pollinators require food, nesting sites, water, and a safe area that will enable them to live in peace. Providing the resources need for pollinator populations doesn’t require as much maintenance as one might imagine. For a program such as this it is the small actions that make the biggest difference, “Plant natives… in your yards, and native flowers in corner lots and at the edges of crop fields… get bee homes… Small actions make a difference when it comes to helping pollinators.” Krakos (2015). -

Guide to Bumble Bees of the Western United States
Bumble Bees of the Western United States By Jonathan Koch James Strange A product of the U.S. Forest Service and the Pollinator Partnership Paul Williams with funding from the National Fish and Wildlife Foundation Executive Editor Larry Stritch, Ph.D., USDA Forest Service Cover: Bombus huntii foraging. Photo Leah Lewis Executive and Managing Editor Laurie Davies Adams, The Pollinator Partnership Graphic Design and Art Direction Marguerite Meyer Administration Jennifer Tsang, The Pollinator Partnership IT Production Support Elizabeth Sellers, USGS Alphabetical Quick Reference to Species B. appositus .............110 B. frigidus ..................46 B. rufocinctus ............86 B. balteatus ................22 B. griseocollis ............90 B. sitkensis ................38 B. bifarius ..................78 B. huntii ....................66 B. suckleyi ...............134 B. californicus ..........114 B. insularis ...............126 B. sylvicola .................70 B. caliginosus .............26 B. melanopygus .........62 B. ternarius ................54 B. centralis ................34 B. mixtus ...................58 B. terricola ...............106 B. crotchii ..................82 B. morrisoni ...............94 B. vagans ...................50 B. fernaldae .............130 B. nevadensis .............18 B. vandykei ................30 B. fervidus................118 B. occidentalis .........102 B. vosnesenskii ..........74 B. flavifrons ...............42 B. pensylvanicus subsp. sonorus ....122 B. franklini .................98 2 Bumble Bees of the