Zoology ABSTRACT Seasonal Variation of Physico-Chemical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cuddalore (Куддалоре) Travel Guide
Cuddalore Travel Guide - http://www.ixigo.com/travel-guide/cuddalore page 1 Max: 35.5°C Min: 25.5°C Rain: 37.7000007629394 When To 5mm Cuddalore Aug Situated in Tamil Nadu, Cuddalore Pleasant weather. Carry Light woollen, VISIT umbrella. is rapidly developing as an Max: 35.0°C Min: Rain: industrial city. An ancient port 25.20000076 58.7000007629394 http://www.ixigo.com/weather-in-cuddalore-lp-1059103 2939453°C 5mm town, it is also home to the second Sep largest beach in Asia. The Jan Pleasant weather. Carry Light woollen, Padaleeswarar Temple, dedicated Famous For : City Pleasant weather. Carry Light woollen. umbrella. Max: Min: Rain: 61.5mm to Lord Shiva, is a massive crowd Max: 29.5°C Min: Rain: 20.70000076 20.7000007629394 34.09999847 24.89999961 puller here. The Gadilam River divides Cuddalore into 2939453°C 53mm 4121094°C 8530273°C 'Old Town and the 'New Town'. The bustling Feb Oct city has a multitude of ancient temples Pleasant weather. Carry Light woollen. Pleasant weather. Carry Light woollen, which attracts a number of tourists all umbrella. Max: Min: Rain: around the year. Dedicate some time to the 30.70000076 21.39999961 6.19999980926513 Max: Min: Rain: 129.0mm 2939453°C 8530273°C 7mm 31.89999961 24.20000076 highly revered and beautiful Sri 8530273°C 2939453°C Paataleeswarar Temple. Don't miss the Mar Nov Pleasant weather. Carry Light woollen. famous Vaishnavite temple of Sri Pleasant weather. Carry Light woollen, Devanathan, situated in Thiruvahindrapura, Max: Min: Rain: umbrella. 32.40000152 23.10000038 14.1000003814697 which is one of the 108 Vaishnavite temples 5878906°C 1469727°C 27mm Max: Min: Rain: 29.79999923 22.89999961 173.600006103515 in India. -

Narrative Notes on Plan Programmes Tam-N
y » I ; ^ t O M i T*' NARRATIVE NOTES ON PLAN PROGRAMMES ANNUAL PLAN 2000-01 STATE PLANNING COMMISSION CHENNAI - 600 005 - 5 * 4 8 2 3 0 < » - 2 5 - TAM-N AUGUST 2000 NARRATIVE NOTES ON PLAN PROGRAMMES 2000-01 NIEPA DC D11079 ' xA^\Q§ i , , .‘♦1 Zi. i-I. Mr:,-, ' 3 )-u o 79 V ^ ' ' Z4* - o 4"* Zc © I CONTENTS Page 1. Crop Husbandry 1 2. Research and Education 25 3. Food, Storage & WareHousing 30 4. Soil & Water Conservation 35 5. Animal Husbandry 41 6. Dairy Developnnent 50 7. Fisheries 53 8. Forests 61 9. Investment in Agri.Financial Institutions 69 10. Co-operation 71 11. Special Programme for Rural Development 75 12. Land Reforms 79 13. Community Development 80 14. Minor Irrigation 83 15. Command Area Development 88 16. Major, Medium Irrigation & Flood Control 90 17. Power Development 103 18. Non-Conventional Sources of Energy 111 19. Industries- Medium and Large 114 20. Village and Small Industries 130 21. Weights and Measures 142 22. Mining and Metallurgical Industries 143 23. Roads and Bridges 145 24. Road and Inland Water Transport 156 25. Scientific Services and Research 158 26. Ecology and Environment 163 27. Secretariat Economic Services 166 28. Tourism 171 29. Economic Advice and Statistics 175 30. Civil Supplies 179 31. General Education 184 CONTENTS—conf. Pagee 32. Technical Education 1988 33. Art and Culture 2011 34. Sports and Youth Services 207)7 35. Medical 21C0 36. Public Health 2188 37. Water Supply and Sanitation 2332 38. Housing 24ft6 39. Urban Development 2551 40. Information and Publicity 2558 41. -

Cuddalore District
DISTRICT DIAGNOSTIC REPORT (DDR) Tamil Nadu Rural Transformation Project Cuddalore District 1 1 DDR - CUDDALORE 2 DDR - CUDDALORE Table of Contents S.No Contents Page No 1.0 Introduction 10 1.1 About Tamil Nadu Rural Transformation Project - TNRTP 1.2 About District Diagnostic Study – DDS 2.0 CUDDALORE DISTRICT 12 2.1 District Profile 3.0 Socio Demographic profile 14 3.1 Population 3.2 Sex Ratio 3.3 Literacy rate 3.4 Occupation 3.5 Community based institutions 3.6 Farmer Producer Organisations (FPOs) 4.0 District economic profile 21 4.1 Labour and Employment 4.2 Connectivity 5.0 GEOGRAPHIC PROFILE 25 5.1 Topography 5.2 Land Use Pattern of the District 5.3 Land types 5.4 Climate and Rainfall 5.5 Disaster Vulnerability 5.6 Soil 5.7 Water Resources 31 DDR - CUDDALORE S.No Contents Page No 6.0 STATUS OF GROUND WATER 32 7.0 FARM SECTOR 33 7.1 Land holding pattern 7.2 Irrigation 7.3 Cropping pattern and Major crops 7.4 Block wise (TNRTP) cropping area distribution 7.5 Prioritization of crops 7.6 Crop wise discussion 8.0 MARKETING AND STORAGE INFRASTRUCTURE 44 9.0 AGRIBUSINESS OPPORTUNITIES 46 10.0 NATIONAL AND STATE SCHEMES ON AGRICULTURE 48 11.0 RESOURCE INSTITUTIONS 49 12.0 ALLIED SECTORS 50 12.1 Animal Husbandry and Dairy development 12.2 Poultry 12.3 Fisheries 12.4 Sericulture 4 DDR - CUDDALORE S.No Contents Page No 13.0 NON-FARM SECTORS 55 13.1 Industrial scenario in the district 13.2 MSME clusters 13.3 Manufacturing 13.4 Service sectors 13.5 Tourism 14.0 SKILL GAPS 65 15.0 BANKING AND CREDIT 67 16.0 COMMODITY PRIORITISATION 69 SWOT ANALYSIS 72 CONCLUSION 73 ANNEXURE 76 51 DDR - CUDDALORE List of Tables Table Number and details Page No Table .1. -

Annexure – 1 List of Tourist Places in Tamil Nadu -..::Tamilnadu Tourism
Annexure – 1 List of Tourist Places in Tamil Nadu Name of Beaches Eco- Tourism Wildlife / Bird Others Art & Culture / Heritage Pilgrim Centers Hills the District (1) (2) Sanctuary (4 & 5) (6) Stations ( 3) Chennai 1.Elliots Beach 1.Guindy, 1.High Court of 1.St. George Fort 1. AshtalakshmiTemple, 2. Marina Beach Children’s Park Madras 2. Ameer Mahal Chennai2.KapaleeswararTemple, 3. Light House 2.SnakePark 2.Madras University 3. VivekanandarIllam Mylapore 3.Parthasarathi Temple, 3.Rippon Building 4.Valluvar Kottam Triplicane 4. TidelPark 5.Gandhi Mandapam 4.Vadapalani Murugan Temple 5.BirlaKolarangam 6.Kamarajar Memorial 5.St.Andru’s Church 6.Lait Kala Academy 7.M.G.R Memorial 6.Santhome Catherdral 7. AnnanagarTower 8.Periyar Memorial 7.Makka Mosque, Thousand Lights 8.Apollo Hospital 9.Connemara public library 8.Shirdi SaibabaTemple, Mylapore 9.SankaraNethralaya 10.Govt. Museum, Egmore 9.KalingambalTemple, Parry’s 10. Adayar cancer 11.Fort Museum 10.Marundeeswarar Temple, Hospital and 12. Kalashethra Tiruvanmiyur Institute 13. Rail Museum, Perambur 11.Jain Temple 11. Vijaya Hospital, 14. Rajaji Hall 12.Iyyappan Vadaplani 15.Anna Square Temple,Mahalingapuram&Annanagar 12.Sankara 16.Barathiyar Memorial 13.Thirumalai TirupattyDevasthanam, NethralayaEye 17. M.G.R. Illam T. Nagar Hospital. 18. Govt. Fine Arts Collage. 14.Buddhavihar, Egmore 13. Adyar 15.Madhiya Kailash Temple, Adyar BaniyanTree 16.RamakrishnaTemple 14. Arvind Eye 17. Velankanni Church, Beasant Nagar Hospital 18.St. George Catherdral 19. BigMosque,Triplicane. Name of Beaches Eco- Tourism Wildlife / Bird Others Art & Culture / Heritage Pilgrim Centers Hills the District Sanctuary Stations Ariyalur 1.Karaivetti 1.Fossile Museum 1.JayankondamPalace 1.Adaikala Madha Shrine, Elakurichi Bird Sanctuary 2. -
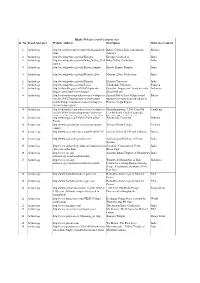
2.Hindu Websites Sorted Category Wise
Hindu Websites sorted Category wise Sl. No. Broad catergory Website Address Description Reference Country 1 Archaelogy http://aryaculture.tripod.com/vedicdharma/id10. India's Cultural Link with Ancient Mexico html America 2 Archaelogy http://en.wikipedia.org/wiki/Harappa Harappa Civilisation India 3 Archaelogy http://en.wikipedia.org/wiki/Indus_Valley_Civil Indus Valley Civilisation India ization 4 Archaelogy http://en.wikipedia.org/wiki/Kiradu_temples Kiradu Barmer Temples India 5 Archaelogy http://en.wikipedia.org/wiki/Mohenjo_Daro Mohenjo_Daro Civilisation India 6 Archaelogy http://en.wikipedia.org/wiki/Nalanda Nalanda University India 7 Archaelogy http://en.wikipedia.org/wiki/Taxila Takshashila University Pakistan 8 Archaelogy http://selians.blogspot.in/2010/01/ganesha- Ganesha, ‘lingga yoni’ found at newly Indonesia lingga-yoni-found-at-newly.html discovered site 9 Archaelogy http://vedicarcheologicaldiscoveries.wordpress.c Ancient Idol of Lord Vishnu found Russia om/2012/05/27/ancient-idol-of-lord-vishnu- during excavation in an old village in found-during-excavation-in-an-old-village-in- Russia’s Volga Region russias-volga-region/ 10 Archaelogy http://vedicarcheologicaldiscoveries.wordpress.c Mahendraparvata, 1,200-Year-Old Cambodia om/2013/06/15/mahendraparvata-1200-year- Lost Medieval City In Cambodia, old-lost-medieval-city-in-cambodia-unearthed- Unearthed By Archaeologists 11 Archaelogy http://wikimapia.org/7359843/Takshashila- Takshashila University Pakistan Taxila 12 Archaelogy http://www.agamahindu.com/vietnam-hindu- Vietnam -

Study of Evaluation of Groundwater in Gadilam Basin Using Hydrogeochemical and Isotope Data
Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data Prasanna M.V1,*, Chidambaram S2, Shahul Hameed A 3, and Srinivasamoorthy K2 1. School of Engineering and Science, Curtin University of Technology, Sarawak, Miri, Malaysia. 2. Department of Earth Sciences, Annamalai University, Annamalainagar, India. 3. Centre for Water Resources Development and Management, Kozhikode, Kerala, India. *Corresponding author’s email. [email protected] Abstract Gadilam river basin has gained its importance due to the presence of Neyveli Lignite open cast mines and other industrial complexes. It is also due to extensive depressurization of Cuddalore aquifer and bore wells for New Veeranam Scheme is constructed in the downstream of the basin. Geochemical indicators of groundwater were used to identify the chemical processes that control hydrogeochemsitry. Chemical parameters of groundwater such as pH, Electrical Conductivity (EC), Total dissolved solids + + + + - (TDS), Sodium (Na ), Potassium (K ), Calcium (Ca ), Magnesium (Mg ), Bicarbonate (HCO3 ), Sulphate - - (SO4 ), Phosphate (PO4 ) and Silica (H4SiO4) were determined. Interpretation of hydrogeochemical data suggests that leaching of ions followed by weathering and anthropogenic impact controls the chemistry of the groundwater. Isotopic study reveals that recharge from meteoric source in sedimentary terrain and rock water interaction with significant evaporation prevails in hard rock region. Keywords: Groundwater, Water chemistry, Stable Isotopes, Thermodynamic stability, Saturation Index 1. Introduction Groundwater is a natural resource, which is being renewed by different process. Geochemical processes occurring within the groundwater and reactions with dissolved minerals have a profound effect on water quality. Hydrogeochemical composition of groundwater can indicate its origin and history of passage through underground materials, which water has been in contact. -

Uzhavar Peruvizha 2013-Cuddalore District
Uzhavar Peruvizha 2013-Cuddalore District ACTION PHOTOGRAPHS Co-Ordinating Centre : KVK, Vridhachalam Reporting period : 14.04.13 to 20.04.13 Date : 14.04.2013 Place : Melalingipattu Scientist : Dr.R.Arunachalam.,Professor and head Activity : Uzhavar Peruvizha - Inaugural Function with Honourable Minister M.C. Sampath Date : 14.04.2013 Place : Melallingipattu Date : 16.04.2013 Place : Karaikadu Scientist: Dr.R.Arunachalam.,P&H Activity: Minister visiting Scientist: Dr.T.Saravanan. Activity: Seed Treatment the exhibition stall. lecture cum demo Date : 17.04.2013 Place : U.Adanur Date : 15.04.2013 Place : Thirumanikuzhi Scientist: Dr.S.Kannan, Activity: Pro tray nursery- Scientist: Dr.T.Saravanan., Activity: Pests and demo disease management in rice- Lecture cum discussion Date: 16.04.2013 Place: Karumbur Date: 17.04.2013 Place:Karuvepilankuruchi Scientist: Th.R.Rajesh Kannan Activity: Demo on latest Scientist: Tmt. G. Meenalakshmi Activity:Soil sample pulse varieties Analysis – lecture cum demo Date : 18.04.2013 Place : Marungur Date : 19.04.2013 Place : Su Keeranur Scientist: Dr.S.Kannan, Activity: Post Harvest Scientist: Dr.S.Kannan, Activity: SSI Techniques - technologies-Lecture Lecture cum demo Date : 20.04.2013 Place : Kilingikuppam Date : 20.04.2013 Place : Kammapuram Scientist: Dr.T.Saravannan, Activity: Pest Management- Scientist: Dr.S.Kannan, Activity: SSI lecture cum technologies-Lecture discussion cum discussion Date : 19.04.2013 Place : Boothambur Date : 19.04.2013 Place : Kandarakottai Scientist: Tmt.G.Meenalakshmi Activity: SRI and KVK Scientist: Th.R.Rajeskannan Activity: SSI technologies Activities – - Discussion Lecture cum discussion Date : 20.04.2013 Place : Dharmanallur Scientist: Dr.S.Kannan, Activity: Lecture cum demo on Value Added technologies Weekly Report on the activities of Block Level Task Force Members / Scientists Sl. -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2009-11. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2011 [Price: Rs. 33.60 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 28A] CHENNAI, WEDNESDAY, JULY 27, 2011 Aadi 11, Thiruvalluvar Aandu–2042 Part II—Section 2 (Supplement) NOTIFICATIONS BY GOVERNMENT AGRICULTURE DEPARTMENT NOTIFICATION OF CROPS, DISTRICT BLOCKS AND FIRKAS FOR KHARIF 2011UNDER NATIONAL AGRICULTURAL INSURANCE SCHEME [G.O. Rt. No. 226, Agriculture (AP1), 12th July 2001, Aani 27, Thiruvalluvar Aandu-2042.] No. II(2)/AG/347/2011. The Tamil Nadu State Level Coordination Committee on Crop Insurance have notified areas in Tamil Nadu for Paddy I (Kar/Kuruvai/Sornawari), Paddy II (Samba/Thalady/Pishanam) and Kharif (Other crops) 2011 season under National Agricultural Insurance Scheme in their Meeting held on 31.05.2011, and its subsequent minutes under reference Government letter No.11255/AP1/2011-3 dated 08.06.2011 of the Department of Agriculture, Secretariat, Fort St.George, Chennai are as below:— ABSTRACT S.No. Name of the crop. No.of Districts. No.of Blocks. No. of Firkas . I Agricultural Crops 1 Paddy-I (Kar/Kuruvai/Sornavari) 28 264 690 2 Paddy-II (Samba/Thaladi/Pishanam) 30 322 906 3 Cholam 14 92 — 4 Cumbu 10 56 — 5 Ragi 7 31 — 6 Maize 22 96 188 7 Redgram 3 31 76 8 Blackgram 7 38 95 9 Greengram 1 7 12 10 Groundnut 28 251 544 11 Gingelly 11 48 — 12 Cotton 19 86 147 13 Sunflower 16 39 .. DTP—II-2 Sup. (28A)—1 [ 1 ] 2 S. No. Name of the crop. -

Tamil Nadu Public Service Commission Bulletin
© [Regd. No. TN/CCN-466/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 196/2009 2015 [Price: Rs. 280.80 Paise. TAMIL NADU PUBLIC SERVICE COMMISSION BULLETIN No. 18] CHENNAI, SUNDAY, AUGUST 16, 2015 Aadi 31, Manmadha, Thiruvalluvar Aandu-2046 CONTENTS DEPARTMENTAL TESTS—RESULTS, MAY 2015 Name of the Tests and Code Numbers Pages. Pages. Second Class Language Test (Full Test) Part ‘A’ The Tamil Nadu Wakf Board Department Test First Written Examination and Viva Voce Parts ‘B’ ‘C’ Paper Detailed Application (With Books) (Test 2425-2434 and ‘D’ (Test Code No. 001) .. .. .. Code No. 113) .. .. .. .. 2661 Second Class Language Test Part ‘D’ only Viva Departmental Test in the Manual of the Firemanship Voce (Test Code No. 209) .. .. .. 2434-2435 for Officers of the Tamil Nadu Fire Service First Paper & Second Paper (Without Books) Third Class Language Test - Hindi (Viva Voce) (Test Code No. 008 & 021) .. .. .. (Test Code 210), Kannada (Viva Voce) 2661 (Test Code 211), Malayalam (Viva Voce) (Test The Agricultural Department Test for Members of Code 212), Tamil (Viva Voce) (Test Code 213), the Tamil Nadu Ministerial Service in the Telegu (Viva Voce) (Test Code 214), Urdu (Viva Agriculture Department (With Books) Test Voce) (Test Code 215) .. .. .. 2435-2436 Code No. 197) .. .. .. .. 2662-2664 The Account Test for Subordinate Officers - Panchayat Development Account Test (With Part-I (With Books) (Test Code No. 176) .. 2437-2592 Books) (Test Code No. 202).. .. .. 2664-2673 The Account Test for Subordinate Officers The Agricultural Department Test for the Technical Part II (With Books) (Test Code No. 190) .. 2593-2626 Officers of the Agriculture Department Departmental Test for Rural Welfare Officer (With Books) (Test Code No. -
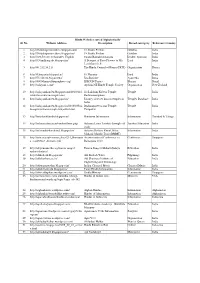
1.Hindu Websites Sorted Alphabetically
Hindu Websites sorted Alphabetically Sl. No. Website Address Description Broad catergory Reference Country 1 http://18shaktipeetasofdevi.blogspot.com/ 18 Shakti Peethas Goddess India 2 http://18shaktipeetasofdevi.blogspot.in/ 18 Shakti Peethas Goddess India 3 http://199.59.148.11/Gurudev_English Swami Ramakrishnanada Leader- Spiritual India 4 http://330milliongods.blogspot.in/ A Bouquet of Rose Flowers to My Lord India Lord Ganesh Ji 5 http://41.212.34.21/ The Hindu Council of Kenya (HCK) Organisation Kenya 6 http://63nayanar.blogspot.in/ 63 Nayanar Lord India 7 http://75.126.84.8/ayurveda/ Jiva Institute Ayurveda India 8 http://8000drumsoftheprophecy.org/ ISKCON Payers Bhajan Brazil 9 http://aalayam.co.nz/ Ayalam NZ Hindu Temple Society Organisation New Zealand 10 http://aalayamkanden.blogspot.com/2010/11/s Sri Lakshmi Kubera Temple, Temple India ri-lakshmi-kubera-temple.html Rathinamangalam 11 http://aalayamkanden.blogspot.in/ Journey of lesser known temples in Temples Database India India 12 http://aalayamkanden.blogspot.in/2010/10/bra Brahmapureeswarar Temple, Temple India hmapureeswarar-temple-tirupattur.html Tirupattur 13 http://accidentalhindu.blogspot.in/ Hinduism Information Information Trinidad & Tobago 14 http://acharya.iitm.ac.in/sanskrit/tutor.php Acharya Learn Sanskrit through self Sanskrit Education India study 15 http://acharyakishorekunal.blogspot.in/ Acharya Kishore Kunal, Bihar Information India Mahavir Mandir Trust (BMMT) 16 http://acm.org.sg/resource_docs/214_Ramayan An international Conference on Conference Singapore -

Monthly Multidisciplinary Research Journal
Vol 7 Issue 2 Nov 2017 ISSN No : 2249-894X ORIGINAL ARTICLE Monthly Multidisciplinary Research Journal Review Of Research Journal Chief Editors Ashok Yakkaldevi Ecaterina Patrascu A R Burla College, India Spiru Haret University, Bucharest Kamani Perera Regional Centre For Strategic Studies, Sri Lanka Welcome to Review Of Research RNI MAHMUL/2011/38595 ISSN No.2249-894X Review Of Research Journal is a multidisciplinary research journal, published monthly in English, Hindi & Marathi Language. All research papers submitted to the journal will be double - blind peer reviewed referred by members of the editorial Board readers will include investigator in universities, research institutes government and industry with research interest in the general subjects. Regional Editor Dr. T. Manichander Sanjeev Kumar Mishra Advisory Board Kamani Perera Delia Serbescu Mabel Miao Regional Centre For Strategic Studies, Sri Spiru Haret University, Bucharest, Romania Center for China and Globalization, China Lanka Xiaohua Yang Ruth Wolf Ecaterina Patrascu University of San Francisco, San Francisco University Walla, Israel Spiru Haret University, Bucharest Karina Xavier Jie Hao Fabricio Moraes de AlmeidaFederal Massachusetts Institute of Technology (MIT), University of Sydney, Australia University of Rondonia, Brazil USA Pei-Shan Kao Andrea Anna Maria Constantinovici May Hongmei Gao University of Essex, United Kingdom AL. I. Cuza University, Romania Kennesaw State University, USA Romona Mihaila Marc Fetscherin Loredana Bosca Spiru Haret University, Romania Rollins College, USA Spiru Haret University, Romania Liu Chen Beijing Foreign Studies University, China Ilie Pintea Spiru Haret University, Romania Mahdi Moharrampour Nimita Khanna Govind P. Shinde Islamic Azad University buinzahra Director, Isara Institute of Management, New Bharati Vidyapeeth School of Distance Branch, Qazvin, Iran Delhi Education Center, Navi Mumbai Titus Pop Salve R. -

Puducherry, Viluppuram, Auroville & Cuddalore
ram . Au ppu rov ilu ille V . C ry u r Tindivanam d e d h a c l u Vanur o d r e u P Viluppuram Auroville Puducherry Panruti S Cuddalore u s n ta la in P ab al le Region SUSTAINABLE REGIONAL PLANNING FRAMEWORK for puducherry, viluppuram, auroville & cuddalore Appendix A Outreach Initiatives February 2012 CONTENTS 1.0 Introduction 5 2.0 Objective of the workshops 12 3.0 Workshop themes 13 4.0 Workshop Schedule and Summary 14 5.0 Issues and recommendations 32 6.0 Public sector participation 48 7.0 Media Outreach 50 1.0 Introduction Puducherry and the adjoining Tamil Nadu region are very closely connected to each other through historical links, culture, religion, language, tourism, trade/business, population, transportation, climate and natural resources such as water bodies, ecosystems, coastline. This tightly knit connection calls for a Regional Plan that would benefit this region not just in the urban areas but also in the adjoining rural areas. With funding assistance from ADEME and endorsement from the Government of Puducherry, INTACH Pondicherry and PondyCAN have embarked upon an initiative to develop a Model Inter-State Sustainable Regional Plan that would help realize the full potential of the region in terms of: sustainable and balanced socio-economic growth, land use development patterns, multimodal connectivity, energy consumption, infrastructure provision and protection of natural resources. Keeping this over-arching goal in mind, the Regional Planning Framework includes the following ‘themes’ that will be addressed in this initial phase- Land Use, Transportation, Energy, and Water. Referred to as the ‘Puducherry – Viluppuram – Auroville- Cuddalore’ (PVAC), the region has been defined as the area generally bounded by the Kaluvelly Tank (Tindivanam taluk of Viluppuram District) on the north, Coromandel Coast on the east, and Perumal Lake (Kurinjipadi taluk of Cuddalore District) on the south.