510(K) SUBSTANTIAL EQUIVALENCE DETERMINATION DECISION SUMMARY ASSAY ONLY TEMPLATE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Exploring Matrix Effects on Binding Properties and Characterization of Cotinine Molecularly Imprinted Polymer on Paper-Based Scaffold
Article Exploring Matrix Effects on Binding Properties and Characterization of Cotinine Molecularly Imprinted Polymer on Paper-Based Scaffold Nutcha Larpant 1, Yaneenart Suwanwong 2, Somchai Boonpangrak 3 and Wanida Laiwattanapaisal 4,5,* 1 Graduate Program in Clinical Biochemistry and Molecular Medicine, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand; [email protected] 2 Department of Clinical Microscopy, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand; [email protected] 3 Center for Research and Innovation, Faculty of Medical Technology, Mahidol University, Nakhon Pathom 73170, Thailand; [email protected] 4 Department of Clinical Chemistry, Faculty of Allied Health Sciences, Chulalongkorn University, Bangkok 10330, Thailand 5 Electrochemistry and Optical Spectroscopy Center of Excellence, Chulalongkorn University, Bangkok 10330, Thailand * Correspondence: [email protected] Received: 22 January 2019; Accepted: 20 March 2019; Published: 26 March 2019 Abstract: Commercially available sorbent materials for solid-phase extraction are widely used in analytical laboratories. However, non-selective binding is a major obstacle for sample analysis. To overcome this problem, molecularly imprinted polymers (MIPs) were used as selective adsorbent materials prior to determining target analysts. In this study, the use of non-covalent molecularly imprinted polymers (MIPs) for cotinine adsorption on a paper-based scaffold was studied. Fiberglass paper was used as a paper scaffold for cotinine-selective MIP adsorption with the use of 0.5% agarose gel. The effects of salt, pH, sample matrix, and solvent on the cotinine adsorption and extraction process were investigated. Under optimal conditions, the adsorption isotherm of synthesized MIPs increased to 125.41 µg/g, whereas the maximum adsorption isotherm of non-imprinted polymers (NIPs) was stable at 42.86 µg/g. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Effect of Varenicline Combined with Medical Management on Alcohol Use Disorder with Comorbid Cigarette Smoking
Table of Contents I. Summary of Changes to the Statistical Plan …………………. 2. II. Summary of Changes to the Protocol………………………… 2. III. Original Protocol at Trial Initiation ………………………… 5. 1 Downloaded From: https://jamanetwork.com/ on 09/28/2021 I. Summary of changes to the statistical analysis plan The original statistical analysis plan is described in the original protocol below beginning on page 20. In the original statistical analysis plan the stratification factor sex was omitted unintentionally from the primary model specification for the main outcome (percent heavy drinking days, PHDD) although sex was identified as a potential moderator in an exploratory aim. At the analysis stage, we included both stratification factors (site and sex) and their interactions with treatment in the model for PHDD consistent with prior hypothesis that men and women differ in their smoking and drinking behavior and treatment response, and with good statistical practice. Baseline percent heavy drinking days was specified as a covariate in the original analysis plan but in response to comments by the statistical reviewer was dropped as a covariate and included in the final mixed model analyses reported in the paper. In order to make the baseline and end-point measures comparable, baseline percent heavy drinking days and end-point percent heavy drinking days were both summarized over 8 week periods. This 8-week duration was pre-planned for the end-point measure but modified from the original intention to use 30 days of the baseline period. Unstructured variance-covariance matrix was used for the errors since variances at baseline and end-point were different. -

OPRM1 Genetic Polymorphisms Are Associated with the Plasma Nicotine Metabolite Cotinine Concentration in Methadone Maintenance Patients: a Cross Sectional Study
Journal of Human Genetics (2013) 58, 84–90 & 2013 The Japan Society of Human Genetics All rights reserved 1434-5161/13 www.nature.com/jhg ORIGINAL ARTICLE OPRM1 genetic polymorphisms are associated with the plasma nicotine metabolite cotinine concentration in methadone maintenance patients: a cross sectional study Yu-Ting Chen1, Hsiao-Hui Tsou2, Hsiang-Wei Kuo1, Chiu-Ping Fang1, Sheng-Chang Wang1, Ing-Kang Ho1,3,4, Yao-Sheng Chang1, Chia-Hui Chen1, Chin-Fu Hsiao2, Hsiao-Yu Wu2, Keh-Ming Lin1, Andrew CH Chen5, Jyy-Jih Tsai-Wu6 and Yu-Li Liu1,7,8 Majority of the heroin-dependent patients smoke cigarettes. Although it has been reported that the OPRM1 genetic polymorphism is associated with the brain mu-opioid receptor binding potential in cigarette smokers, there is no direct evidence showing the impact of plasma cotinine, a nicotine metabolite, on treatment responses to methadone maintenance treatment (MMT). In this study, we aimed to test the hypothesis that the genetic polymorphisms in the OPRM1 are associated with the methadone treatment responses and the severity of cigarette smoking directly measured by the plasma concentration of cotinine in a Taiwanese MMT cohort. Fifteen OPRM1 single-nucleotide polymorphisms (SNPs) were selected and genotyped on DNA samples of 366 MMT patients. Plasma concentrations of cotinine were measured by cotinine enzyme-linked immunosorbent assay. The plasma cotinine concentration had positive correlation with concentrations of methadone (P ¼ 0.042) and its metabolite 2-ethylidene-1,5-dimethyl-3,3-diphenyl-pyrrolidine (P ¼ 0.037). Methadone treatment non-responders, defined by a positive urine morphine test, had a higher plasma concentration of cotinine (P ¼ 0.005), but a lower plasma concentration-to- dose ratio of both R- and S-methadone (P ¼ 0.001 and 0.012, respectively) than the responders. -

Nicotine Metabolism and CYP2D6 Phenotype in Smokers
Vol. 10, 261–263, March 2001 Cancer Epidemiology, Biomarkers & Prevention 261 Short Communication Nicotine Metabolism and CYP2D6 Phenotype in Smokers Neil E. Caporaso,1 Caryn Lerman, Janet Audrain, CYP2D6 (debrisoquine hydroxylase) has been studied as a Neil R. Boyd, David Main, Haleem J. Issaq, putative lung cancer susceptibility factor, and it has been pro- Bill Utermahlan, Roni T. Falk, and Peter Shields posed that this enzyme may contribute to the metabolism of Division of Cancer Epidemiology and Genetics, National Cancer Institute, nicotine, thereby influencing the amount a person smokes and Bethesda, Maryland 20892 [N. E. C., R. T. F.]; Lombardi Cancer Research the delivery of carcinogens. Some in vitro (1) and population Center, Georgetown University, Washington, D.C. 20007 [C. L., J. A., (2) studies have suggested a role for CYP2D6 in nicotine N. R. B., D. M.]; Laboratory of Chemical Synthesis and Analysis, Frederick metabolism, although recent work indicates CYP2A6 is the Cancer Research Center, Frederick, Maryland 21701 [H. J. I., B. U.]; and Laboratory of Human Carcinogenesis, Division of Cancer Etiology, NIH, most important P-450 in nicotine metabolism (3, 4). The National Cancer Institute, Bethesda, Maryland 20892 [P. S.] CYP2D6 MP2 is determined by administering DM (5) and determining the ratio of urinary metabolites. A low ratio of unchanged drug:metabolite identifies extensive metabolizers, Abstract whereas PMs exhibit a high ratio. The hypothesis that a poly- We tested the hypothesis that the polymorphic enzyme morphic gene influences nicotine disposition is important be- CYP2D6 is related to nicotine metabolism in 261 healthy cause addicted smokers engage in smoking behavior in such a subjects enrolling in a smoking cessation clinic. -
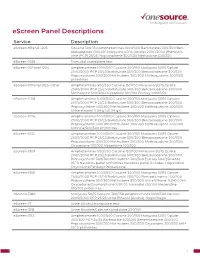
Escreen Panel Descriptions
eScreen Panel Descriptions Service Description eScreen-9Panel-1205 Cocaine 300/150 Amphetamines 1000/500 Barbiturates 300/300 Ben- zodiazepines 300/300 Marijuana 50/15 Opiates 2000/2000 Phencycli- dine (PCP) 25/25 Propoxyphene 300/300 Methadone 300/300 eScreen-1065 Tramadol standalone test eScreen-10Panel-1204 Amphetamines 1000/500 Cocaine 300/150 Marijuana 50/15 Opiate 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone 300/300 Methaqualone 300/300 pH/nitrites eScreen-10Panel-3125-Other Amphetamines 500/250 Cocaine 150/100 Marijuana 50/15 Opiate 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Methadone 300/300 Oxycodone 100/100 Ecstasy 1000/250 eScreen-1208 Amphetamines 1000/500 Cocaine 300/150 Marijuana 50/15 Opiates 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone 300/300 Methaqualone 300/300 Urine ethanol 0.04 g%/0.04 g% eScreen-1224 Amphetamines 1000/500 Cocaine 300/150 Marijuana 50/15 Opiates 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone 300/300 Methaqualone 300/300 Cotinine 500/500 pH/nitrites eScreen-1232 Amphetamines 1000/500 Cocaine 300/150 Marijuana 50/15 Opiate 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone 300/300 Methaqualone 300/300 Oxycodone 100/100 Meperidine 100/100 eScreen-1309 Amphetamines 500/250 Cocaine 150/100 Marijuana 50/15 Opiate 2000/2000 PCP 25/25 Barbiturate 300/300 Benzodiazepine 300/300 Propoxyphene 300/300 Methadone -

Chronic Smokeless Tobacco Consumption Contributes to the Development of Renal Diseases in the Human Male Volunteers
Journal of Analytical & Pharmaceutical Research Research Article Open Access Chronic smokeless tobacco consumption contributes to the development of renal diseases in the human male volunteers Abstract Volume 7 Issue 6 - 2018 Smokeless tobacco may able to induce microalbuminuria. The present study S Fareeda Begum,1 G Nagajothi,2 K investigates the relation between nicotine and cotinine with renal function in gutkha Swarnalatha,1 C Suresh Kumar,1 Narendra and khaini users. Methods: The levels of nicotine and cotinine were estimated by HPLC 1 methods and other urine variables were detected by spectrophotometric methods. Maddu 1 Current smokeless tobacco users have shown that significantly elevated levels of Department of Biochemistry, Sri Krishnadevaraya University, India nicotine, cotinine, and epinephrine excretion in the urine than non-tobacco users. 2Department of Corporate Secretaryship, Queen Mary’s Renal function was assessed by glomerular filtration rate (GFR), levels of urea, and College (Autonomous), India creatinine. Among the kidney function measures that we examined, microalbuminuria, decreased glomerular filtration rate, and creatinine clearance were found associated Correspondence: Narendra Maddu, Assistant Professor, with gutkha and khaini users. Significantly decreased proteinuria, urea and increased Department of Biochemistry, Sri Krishnadevaraya University, levels of uric acid and creatinine excretion with the concomitant increase in plasma Ananthapuramu-515003, Andhra Pradesh, India, Tel 91+ total proteins, urea, and decreased uric acid levels were observed in the group I and 9441983797, Email group II users compared to group III users. The products of smokeless tobacco are regarded as good predictors of assessing the free radical levels in the cells. The active Received: June 01, 2018 | Published: November 26, 2018 markers of nitroxidative stress have been elevated progressively with the uptake of nicotine and exposure. -

Ketamine Homogeneous Enzyme Immunoassay (HEIA™)
Ketamine Homogeneous Enzyme Immunoassay (HEIA™) Exclusively from Immunalysis Formula: C13H16CINO Semi-Quantitative or Qualitative Testing Systematic Name: (RS)- 2- (2- chlorophenyl)- 2- (methylamino)cyclohexanone Accurate and reliable Brand Names: Ketanest®, Ketaset®, Ketalar® Ready to use About Ketamine: Ketamine is an anesthetic agent used in the United States since 1972 for veterinary and pediatric medicine. It is also used in the treatment of depression and postoperative pain management. However, in recent years it has gained popularity as a street drug used at clubs and raves due to its hallucinogenic effects. Administration: Oral; intravenous; intramuscular; insufflation Elimination: Ketamine metabolizes by N-demethylation to Norketamine and further dehydrogenates to Dehydronorketamine. After 72 hours of a single dose, 2.3% of Ketamine is unchanged, 1.6% is Norketamine, 16.2% is Dehydronorketamine, and 80% is hydroxylated derivatives of Ketamine.1,2 Abuse Potential: An overdose can cause unconsciousness and dangerously slowed breathing. 1) R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, Fourth Edition, p. 412-414. 2) K. Moore, J.Skerov, B.Levine, and A.Jacobs, Urine Concentrations of Ketamine and Norketamine Following Illegal Consumption, J.Anal, Toxicol. 25: 583-588 (2001). Ketanest® is a registered trademark of Pfizer, Inc., Ketaset® is a trademark of ZOETIS W LLC., Ketalar® is a trademark of PAR STERILE PRODUCTS, LLC. Tel 909.482.0840 | Toll Free 888.664.8378 | Fax 909.482.0850 ISO13485:2003 www.immunalysis.com CERTIFIED -

Cotinine ELISA 4
3. Disposable pipette tips Cotinine ELISA 4. ELISA reader capable of reading absorbance at 450 nm 5. Absorbance paper or paper towel 7015-47 6. Graph paper RUO 96 Tests PRECAUTION 1. Potential biohazardous materials: The calibrator and controls contain human source components which have Test Cotinine been tested and found non- reactive for hepatitis B surface antigen as well as Enzyme Linked HIV antibody with FDA licensed reagents. However, as there is no test Method Immunosorbent ELISA method that can offer complete assurance that HIV, Hepatitis B virus or other infectious agents are absent, these reagents should be handled at the Principle Direct ELISA Biosafety Level 2, as recommended in the Centers for Disease Sample 10 µl (diluted) Control/National Institutes of Health manual, "Biosafety in Microbiological Sensitivity 1 ng/ml and Biomedical Laboratories." 1984 Total Time ~ 90 min 2. This test kit is designed for research use only. 12 Months from the 3. Do not pipette by mouth. Do not smoke, eat, or drink in the areas in which Shelf Life manufacturing date specimens or kit reagents are handled. 4. The components in this kit are intended for use as an integral unit. The components of different lots should not be mixed. 5. It is recommended that serum samples be run in duplicate. 6. Optimal results will be obtained by strict adherence to this protocol. INTENDED USE Accurate and precise pipetting, as well as following the exact time and The Diagnostic Automation Inc. (DAI) Cotinine ELISA Kit is intended for the temperature requirements prescribed are essential. Any deviation from this measurement of Cotinine in serum and urine. -

Drug Plasma Half-Life and Urine Detection Window | January 2019
500 Chipeta Way | Salt Lake City, UT 84108-1221 Phone: (800) 522-2787 | Fax: (801) 583-2712 www.aruplab.com | www.arupconsult.com DRUG PLASMA HALF-LIFE AND URINE DETECTION WINDOW | JANUARY 2019 URINE- PLASMA DRUG, DRUG METABOLITE(S)* COMMON TRADE AND STREET NAMES, NOTES DETECTION HALF-LIFEt WINDOWt STIMULANTS Benzedrine, dexedrine, Adderall, Vyvanse, speed; could be methamphetamine Amphetamine 7–34 hours 1–5 days metabolite; if so, typically < 30 percent of parent Cocaine Coke, crack; parent drug rarely observed due to short half-life 0.7–1.5 hours < 1 day Benzoylecgonine Cocaine metabolite 5.5–7.5 hours 1–2 days Desoxyn, methedrine, Vicks inhaler (D- and L-isomers not resolved; low concentrations Methamphetamine expected if the source is Vicks); selegeline (Atapryl, Carbex, Eldepryl, Zelapar) 6–17 hours 1–5 days metabolite Methylenedioxyamphetamine (MDA) MDA 11–17 hours 1–3 days Methylenedioxyethylamphetamine (MDEA) MDEA, MDE, Eve 6–11 hours 1–3 days Methylenedioxymethamphetamine (MDMA) MDMA, XTC, ecstasy, Molly 6–10 hours 1–3 days Methylphenidate Ritalin, Concerta, Focalin, Metadate, Methylin 1.4–4.2 hours < 1 day Ritalinic acid Methylphenidate metabolite 1.8–2.5 hours < 1 day Phentermine Adipex-P, Lomaira, Qsymia 19–24 hours 1–5 days OPIOIDS Buprenorphine Belbuca, Buprenex, Butrans, Suboxone, Subutex, Sublocade, Zubsolv 26–42 hours 1–7 days Norbuprenorphine, Glucuronides Buprenorphine metabolites 15–150 hours 1–14 days Included in many preparations; morphine metabolite; may be a contaminant if < 2 Codeine 1.9–3.9 hours 1–3 days percent of morphine Fentanyl Actiq, Duragesic, Fentora, Lazanda, Sublimaze, Subsys, Ionsys 3–12 hours 1–3 days Norfentanyl Fentanyl metabolite 9–10 hours 1–3 days Heroin Diacetylmorphine, dope, smack, dust; parent drug not detected. -

Smoking Cessation Prior to Elective Surgery
2018 OHP Guidelines: Verification of Cessation Smoking Cessation Prior to Elective Surgery Verification of Surgery Smoking Status Cessation Cessation Scheduler Cotinine Current Smoker Options -HPCP code G0432 The scheduler Tobacco Use - Refer to Program -urine or serum sends relevant - Refer to PCP -if positive or if using chart notes and Past 30 days - Do it yourself NRT use one of these PA (prior alternate tests: Anabasine authorization) Recent Quit -HCPC code G0480 request to health Exhaled CO Tobacco Use plan along with: -CPT code 94250 -length of abstinence Past year -on-site equipment -negative lab results -for patients who have quit smoking for at Remote Quit least one year, an Tobacco Use attestation is Over 1 year ago sufficient. Verification of Cessation: Timing issues Verification of abstinence is required for at least four weeks prior to the procedure and requires objective evidence of abstinence from smoking prior to the procedure, which raises timing issues: Procedures Abstinence Requirement Testing required Elective surgical procedures are 1 month One test (urine or serum) 1 month defined as those which are flexible prior to request which starts the in their scheduling because the timeline. If the scheduled date of condition does not pose an procedure is several weeks out, an imminent threat nor does it require additional testing approximately a immediate attention within one week before procedure. month. Lung volume reduction surgery, 6 months Testing 3-4 times during the six bariatric surgery, erectile months is sufficient, again with dysfunction surgery, and spinal evidence of testing at the beginning fusion have 6-month smoking which starts the timeline and abstinence requirements approximately a week before surgery Reproductive procedures (i.e., for contraceptive purposes), cancer-related and diagnostic procedures are excluded from this guideline. -

ARK™ Ketamine Assay Package Insert
The ARK Ketamine Assay provides only a preliminary analytical test result. A more specific alternative chemical method must be used in order to obtain a confirmed positive analytical result. Gas Chromatography/Mass Spectrometry (GC/MS) or Liquid Chromatography/tandem Mass Spectrometry (LC-MS/MS) is the preferred confirmatory method. Clinical consideration and professional judgment should be exercised with any drug test result, particularly when the For Criminal Justice and Forensic Use Only preliminary test result is positive. 3 SUMMARY AND EXPLANATION OF THE TEST Ketamine ((+/-)-2-(2-chlorophenyl)-2-(methylamino)cyclohexanone) is a synthetic, non- RK™ Ketamine Assay barbiturate and rapid-acting general anesthetic that is indicated for use in both human and A veterinary surgical procedures.1,2 This ARK Diagnostics, Inc. package insert for the ARK Ketamine Assay must be read prior to use. Package insert instructions must be followed accordingly. The assay provides a simple Ketamine is a Schedule III substance under the United States Controlled Substances Act for and rapid analytical screening procedure for detecting ketamine in urine. Reliability of the its potential for abuse and risk of dependence. Ketamine is structurally and pharmacologically assay results cannot be guaranteed if there are any deviations from the instructions in this similar to phencyclidine (PCP), but is less potent, has a faster onset and shorter duration of package insert. action relative to PCP. Ketamine produces a variety of symptoms including, but not limited to anxiety, dysphoria, disorientation, insomnia, flashbacks, hallucinations, and psychotic episodes.1,3 CUSTOMER SERVICE Following administration in humans, ketamine is N-demethylated by liver microsomal ARK Diagnostics, Inc.