Impedance Spectroscopy and NMR Studies of Fast Ion Conducting Chalcogenide Glasses Hitendra Kumar Patel Iowa State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mathieu Deflem
Curriculum Vitae Mathieu Deflem (August 2021) University of South Carolina Department of Sociology 911 Pickens Street ColumBia, SC 29208 [email protected] (803) 777 3123 www.mathieudeflem.net ACADEMIC EMPLOYMENT 2002– Professor (since 2010), Associate Professor (2005–2010), Assistant Professor (2002–2005), Department of Sociology, University of South Carolina, ColumBia, SC. 1997–2002 Assistant Professor of Sociology, Department of Sociology and Anthropology, Purdue University, West Lafayette, IN. 1996–1997 Visiting Assistant Professor of Sociology and Law and Society, Department of Anthropology & Sociology, Kenyon College, GamBier, OH. 1989–1996 Pre-doctoral positions: Research Assistant (1992–1995), Teaching Assistant (1995), Instructor (1996), Department of Sociology, University of Colorado, Boulder, CO; Assistant (1989–1992), Afdeling Strafrecht, Strafvordering en Criminologie (Department of Criminal Law, Criminal Procedure, and Criminology), Katholieke Universiteit te Leuven, Belgium. EDUCATION 1996 Ph.D. Sociology, University of Colorado, Boulder, CO. Dissertation: “Borders of Police Force: Historical Foundations of International Policing Between Germany and the United States.” 1990 M.A. Sociology of Developing Societies, University of Hull, England. Thesis: “Processual SymBolic Analysis in the Work of Victor W. Turner.” 1987 Special Diploma Social and Cultural Anthropology (M.A. equivalent), Katholieke Universiteit te Leuven, Belgium. Thesis: “Antropologie van de Ruimte” (Dutch: “The Anthropology of Space”). 1986 Licentiate -

Commercial Radio Members
Commercial Radio Members As of 11/18/2020 WARQ-FM & HD2 (Alpha) WCKN-FM (SAGA) WDAR-FM (iHeart) Rock Country Hip Hop & R&B Mike Hartel Paul O’Malley Jimmy Feuger General Manager President-General Manager General Manager PO Box 9127 2294 Clements Ferry Rd. 181 East Evans St. Ste. 311 Columbia, SC 29290 Charleston, SC 29492 Florence, SC 29506 (803) 776-1013, voice (843) 972-1100, voice (843) 667-4600, voice www.warq.com www.kickin925.com www.sunny1055online.com WAVF-FM (SAGA) WCOS-AM (iHeart) WDKD-AM (Community) Soft Rock Sports Talk Adult Hits Paul O’Malley Ron Hill Wayne Mulling President-General Manager General Manager General Manager 2294 Clements Ferry Rd. 316 Greystone Blvd. PO Box 1269 Charleston, SC 29492 Columbia, SC 29210 Sumter, SC 29151 (843) 972-1100, voice (803) 343-1100, voice (803) 775-2321, voice www.1017chuckfm.com www.1400theteam.com www.cbpeedee/frank971.com WDSC-AM (iHeart) WBCU-AM WCOS-FM & HD2 (iHeart) Sports Country Country Jimmy Feuger Chris Woodson Ron Hill General Manager General Manager General Manager 181 East Evans St. Ste. 311 210 E. Main St. 316 Greystone Blvd. Florence, SC 29506 Union, SC 29379 Columbia, SC 29210 (843) 667-4600, voice (864) 427-2411, voice (803) 343-1100, voice www.sportsconnection800.ihear www.wbcuradio.com www.wcosfm.com t. com WCAM-AM WCRE-AM WDXY-AM (Community) Adult Standards Oldies NewsTalk Chris Johnson Jane Pigg Wayne Mulling General Manager General Manager General Manager PO Box 753 PO Box 160 PO Box 1269 Camden, SC 29021 Cheraw, SC 29520 Sumter, SC 29151 (803) 438-9002, voice (843) 537-7887, voice (803) 775-2321, voice www.kool1027.com www.myfm939.com www.commbroadcasters.com WEGX-FM (iHeart) WFBC-HD2 (Entercom) WGFG-FM (Community) Country Urban Rock Country Jimmy Feuger Steve Sinicropi Wayne Mulling General Manager General Manager General Manager 181 East Evans St. -

Stations Monitored
Stations Monitored 10/01/2019 Format Call Letters Market Station Name Adult Contemporary WHBC-FM AKRON, OH MIX 94.1 Adult Contemporary WKDD-FM AKRON, OH 98.1 WKDD Adult Contemporary WRVE-FM ALBANY-SCHENECTADY-TROY, NY 99.5 THE RIVER Adult Contemporary WYJB-FM ALBANY-SCHENECTADY-TROY, NY B95.5 Adult Contemporary KDRF-FM ALBUQUERQUE, NM 103.3 eD FM Adult Contemporary KMGA-FM ALBUQUERQUE, NM 99.5 MAGIC FM Adult Contemporary KPEK-FM ALBUQUERQUE, NM 100.3 THE PEAK Adult Contemporary WLEV-FM ALLENTOWN-BETHLEHEM, PA 100.7 WLEV Adult Contemporary KMVN-FM ANCHORAGE, AK MOViN 105.7 Adult Contemporary KMXS-FM ANCHORAGE, AK MIX 103.1 Adult Contemporary WOXL-FS ASHEVILLE, NC MIX 96.5 Adult Contemporary WSB-FM ATLANTA, GA B98.5 Adult Contemporary WSTR-FM ATLANTA, GA STAR 94.1 Adult Contemporary WFPG-FM ATLANTIC CITY-CAPE MAY, NJ LITE ROCK 96.9 Adult Contemporary WSJO-FM ATLANTIC CITY-CAPE MAY, NJ SOJO 104.9 Adult Contemporary KAMX-FM AUSTIN, TX MIX 94.7 Adult Contemporary KBPA-FM AUSTIN, TX 103.5 BOB FM Adult Contemporary KKMJ-FM AUSTIN, TX MAJIC 95.5 Adult Contemporary WLIF-FM BALTIMORE, MD TODAY'S 101.9 Adult Contemporary WQSR-FM BALTIMORE, MD 102.7 JACK FM Adult Contemporary WWMX-FM BALTIMORE, MD MIX 106.5 Adult Contemporary KRVE-FM BATON ROUGE, LA 96.1 THE RIVER Adult Contemporary WMJY-FS BILOXI-GULFPORT-PASCAGOULA, MS MAGIC 93.7 Adult Contemporary WMJJ-FM BIRMINGHAM, AL MAGIC 96 Adult Contemporary KCIX-FM BOISE, ID MIX 106 Adult Contemporary KXLT-FM BOISE, ID LITE 107.9 Adult Contemporary WMJX-FM BOSTON, MA MAGIC 106.7 Adult Contemporary WWBX-FM -

Truth in Spending Report
City of Columbia Truth in Spending Detail Report - Sorted by Check Date Date Range: 05/01/2019 - 05/31/2019 Payee Name Date Amount City Division Check Description Budget Category AT AND T CORP 05/02/2019 137.38 Fire Suppression 256126794 Internet ENVIRONMENTAL EDUCATION ASSOCI 05/02/2019 1,000.00 Engineering Storm Water Imp "Palmetto" Sponsorship level f Advertising GOVERNMENT FINANCE OFFICERS AS 05/02/2019 100.00 Admin-Chief Financial Officer GFOASC 2019 SPRING CONFERENCE Employee Training & Prof Dev. IAFC REGISTRATION CENTER 05/02/2019 250.00 Fire Administration IAFC Membership Dues For Chief Membership And Dues INTERNATIONAL ECONOMIC DEVELOP 05/02/2019 630.00 Economic Development Registration Fees for Kay Hamp Employee Training & Prof Dev. MOTOROLA INC 05/02/2019 68.71 Streets Street & Sidewalk Rpr 6530AF Radio Maintenance PROTOW OF COLUMBIA INC 05/02/2019 160.00 Police Operations Travel - Lodging Travel - Lodging PROTOW OF COLUMBIA INC 05/02/2019 194.00 Police Operations Travel - Lodging Travel - Lodging SC DEPT OF LABOR LICENSING & R 05/02/2019 30.00 Utilities Metro Wastewater Plt TYRONE SALTERS Membership And Dues SC DEPT OF LABOR LICENSING & R 05/02/2019 30.00 Utilities Water Dist & Maint ROBERT ALLISON Employee Training & Prof Dev. SC DEPT OF LABOR LICENSING & R 05/02/2019 30.00 Utilities Water Dist & Maint CRAIG W. BOYKIN Employee Training & Prof Dev. SC DEPT OF LABOR LICENSING & R 05/02/2019 50.00 Utilities Water Dist & Maint DEVONTE BROOKS Employee Training & Prof Dev. SC DEPT OF LABOR LICENSING & R 05/02/2019 30.00 Utilities Water Dist & Maint BARRETT D. -

Order and Consent Decree
Federal Communications Commission DA 16-3 Before the Federal Communications Commission Washington, DC 20554 In the Matter of ) ) File No.: EB-IHD-14-000151152 Radio License Holding CBC, LLC ) Acct. No.: 201632080003 ) FRN: 0019721638 Former Licensee of Station WOKQ(FM), ) Facility ID No.: 22887 Dover, New Hampshire1; and ) ) Cumulus Radio Corporation ) FRN: 0001595214 ) ORDER Adopted: January 7, 2016 Released: January 7, 2016 By the Chief, Enforcement Bureau: 1. The Enforcement Bureau (Bureau) of the Federal Communications Commission (Commission) has entered into a Consent Decree to resolve its investigation into whether Radio License Holding CBC, LLC (Radio License), and Radio License’s parent, Cumulus Radio Corporation (CRC), broadcast announcements on radio station WOKQ(FM), Dover, New Hampshire (Station), without adequate sponsorship disclosure in violation of the Commission’s sponsorship identification laws. 2. The Commission’s sponsorship identification laws protect consumers and promote fair competition by requiring that the sponsors of paid programming material be clearly identified. Those laws are based on the principle that listeners and viewers are entitled to know who seeks to persuade them. The disclosures required by those laws provide listeners and viewers with information concerning the source of material in order to prevent misleading or deceiving those listeners and viewers. Enforcement of the sponsorship identification laws also protects fair competition among advertisers. We seek to prevent sponsors from gaining unfair advantage by paying stations to present promotional messages without appropriate disclosures, while their competitors observe the rules and present their content as properly acknowledged commercial advertisements. 3. The Bureau investigated a complaint that the Station broadcast announcements supporting a hydro-electronic energy project in New Hampshire without disclosing the identity of the company that sponsored the announcements. -
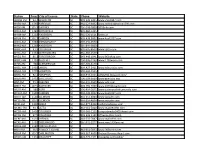
Complete Radio Roster
Station Freq City of License State Phone Website WAAW-FM 94.7 WILLISTON SC 803-649-6405 www.shout947.com WABV-AM 1590 ABBEVILLE SC 864-223-9402 www.radioinspiracion1590.com WOSF-FM 105.3 GAFFNEY SC 704-548-7800 1053rnb.com WAGS-AM 1380 BISHOPVILLE SC 803-484-5415 WAIM-AM 1230 ANDERSON SC 864-226-1511 waim.us WPUB-FM 102.7 CAMDEN SC 803-438-9002 www.kool1027.com WALD-AM 1080 JOHNSONVILLE SC 803-939-9530 WANS-AM 1280 ANDERSON SC 864-844-9009 WARQ-FM 93.5 COLUMBIA SC 803-695-8600 www.q935.com WASC-AM 1530 SPARTANBURG SC 864-585-1530 WKZQ-FM 96.1 FORESTBROOK SC 843-448-1041 www.961wkzq.com WAVO-AM 1150 ROCK HILL SC 704-596-1240 www.1150wavo.com WAZS-AM 980 SUMMERVILLE SC 704-405-3170 WBCU-AM 1460 UNION SC 864-427-2411 www.wbcuradio.com WHGS-AM 1270 HAMPTON SC 803-943-5555 WBHC-FM 92.1 HAMPTON SC 803-943-5555 allhits921.blogspot.com/ WBLR-AM 1430 BATESBURG SC 706-309-9609 www.gnnradio.org WBT-FM 99.3 CHESTER SC 704-374-3500 www.wbt.com WNKT-FM 107.5 EASTOVER SC 803-796-7600 www.1075thegame.com WULR-AM 980 YORK SC 336-434-5025 www.cadenaradialnuevavida.com WCAM-AM 1590 CAMDEN SC 803-438-9002 www.kool1027.com WAHT-AM 1560 CLEMSON SC 864-654-4004 www.wccpfm.com WCCP-FM 105.5 CLEMSON SC 864-654-4004 www.wccpfm.com WCKI-AM 1300 GREER SC 864-877-8458 catholicradioinsc.com WCMG-FM 94.3 LATTA SC 843-661-5000 www.magic943fm.com WCOS-AM 1400 COLUMBIA SC 803-343-1100 foxsportsradio1400.iheart.com WCOS-FM 97.5 COLUMBIA SC 803-343-1100 975wcos.iheart.com WCRE-AM 1420 CHERAW SC 843-537-7887 www.myfm939.com WCRS-AM 1450 GREENWOOD SC 864-941-9277 www.wcrs1450am.net -

Federal Communications Commission DA 11-1546 Before the Federal
Federal Communications Commission DA 11-1546 Before the Federal Communications Commission Washington, D.C. 20554 In the Matter of ) ) Existing Shareholders of Cumulus ) BTC-20110330ALU, et al., Media, Inc. (Transferors) ) BTCH-20110331AIF, et al., and ) BTCH-20110331 AJF, et al., Existing Shareholders of Citadel ) BTCH-20110331AJN Broadcasting Corporation (Transferors) ) BTC-20110331AJO and ) BTCFT-20110331AKE, et al., New Shareholders of Cumulus Media, Inc. ) BTC-20110330ADE, et al., (Transferees) ) BTC-20110330ALJ, et al., ) BTCH-20110330ALM, et al., For Consent to Transfers of Control ) BTCH-20110330ALO, et al., ) BTCH-20110330AYC ) BTC-20110330AYD ) BTC-20110330AYF, et al., ) BTC-20110331AAA, et al., ) BTC-20110331AEV, ) BTC-20110331AEU ) BTC-20110331AEW ) BTCH-20110331AEX ) BTC-20110331AHZ, et al., ) BTCFT-20110510ADO, et al., ) Existing Shareholders of Cumulus ) BALH-20110331AID, et al., Media, Inc. ) BAL-20110331AJP, et al., (Assignors) ) BALH-20110331AJZ and ) BAL-20110331AKA Existing Shareholders of Citadel ) Broadcasting Corporation ) (Assignors) ) and ) Volt Radio, LLC, as Trustee ) (Assignee) ) ) For Consent to Assignment of Licenses ) MEMORANDUM OPINION AND ORDER Adopted: September 14, 2011 Released: September 14, 2011 By the Chief, Media Bureau: Federal Communications Commission DA 11-1546 I. INTRODUCTION 1. The Media Bureau (“Bureau”) has under consideration the captioned transfer and assignment applications (the “Applications”), as amended,1 in connection with a proposed transaction whereby a wholly-owned subsidiary of -

Stations Monitored
Stations Monitored Call Letters Market Station Name Format WAPS-FM AKRON, OH 91.3 THE SUMMIT Triple A WHBC-FM AKRON, OH MIX 94.1 Adult Contemporary WKDD-FM AKRON, OH 98.1 WKDD Adult Contemporary WRQK-FM AKRON, OH ROCK 106.9 Mainstream Rock WONE-FM AKRON, OH 97.5 WONE THE HOME OF ROCK & ROLL Classic Rock WQMX-FM AKRON, OH FM 94.9 WQMX Country WDJQ-FM AKRON, OH Q 92 Top Forty WRVE-FM ALBANY-SCHENECTADY-TROY, NY 99.5 THE RIVER Adult Contemporary WYJB-FM ALBANY-SCHENECTADY-TROY, NY B95.5 Adult Contemporary WPYX-FM ALBANY-SCHENECTADY-TROY, NY PYX 106 Classic Rock WGNA-FM ALBANY-SCHENECTADY-TROY, NY COUNTRY 107.7 FM WGNA Country WKLI-FM ALBANY-SCHENECTADY-TROY, NY 100.9 THE CAT Country WEQX-FM ALBANY-SCHENECTADY-TROY, NY 102.7 FM EQX Alternative WAJZ-FM ALBANY-SCHENECTADY-TROY, NY JAMZ 96.3 Top Forty WFLY-FM ALBANY-SCHENECTADY-TROY, NY FLY 92.3 Top Forty WKKF-FM ALBANY-SCHENECTADY-TROY, NY KISS 102.3 Top Forty KDRF-FM ALBUQUERQUE, NM 103.3 eD FM Adult Contemporary KMGA-FM ALBUQUERQUE, NM 99.5 MAGIC FM Adult Contemporary KPEK-FM ALBUQUERQUE, NM 100.3 THE PEAK Adult Contemporary KZRR-FM ALBUQUERQUE, NM KZRR 94 ROCK Mainstream Rock KUNM-FM ALBUQUERQUE, NM COMMUNITY RADIO 89.9 College Radio KIOT-FM ALBUQUERQUE, NM COYOTE 102.5 Classic Rock KBQI-FM ALBUQUERQUE, NM BIG I 107.9 Country KRST-FM ALBUQUERQUE, NM 92.3 NASH FM Country KTEG-FM ALBUQUERQUE, NM 104.1 THE EDGE Alternative KOAZ-AM ALBUQUERQUE, NM THE OASIS Smooth Jazz KLVO-FM ALBUQUERQUE, NM 97.7 LA INVASORA Latin KDLW-FM ALBUQUERQUE, NM ZETA 106.3 Latin KKSS-FM ALBUQUERQUE, NM KISS 97.3 FM -

Complete Report
Acknowledgments FMC would like to thank Jim McGuinn for his original guidance on playlist data, Joe Wallace at Mediaguide for his speedy responses and support of the project, Courtney Bennett for coding thousands of labels and David Govea for data management, Gabriel Rossman, Peter DiCola, Peter Gordon and Rich Bengloff for their editing, feedback and advice, and Justin Jouvenal and Adam Marcus for their prior work on this issue. The research and analysis contained in this report was made possible through support from the New York State Music Fund, established by the New York State Attorney General at Rockefeller Philanthropy Advisors, the Necessary Knowledge for a Democratic Public Sphere at the Social Science Research Council (SSRC). The views expressed are the sole responsibility of its author and the Future of Music Coalition. © 2009 Future of Music Coalition Table of Contents Introduction ..................................................................................................................... 4 Programming and Access, Post-Telecom Act ........................................................ 5 Why Payola? ........................................................................................................... 9 Payola as a Policy Problem................................................................................... 10 Policy Decisions Lead to Research Questions...................................................... 12 Research Results .................................................................................................. -

Commission Meeting Agenda a Public Notice of the Federal Communications Federal Communications Commission Commission 445 12Th Street, S.W
Commission Meeting Agenda A Public Notice of the Federal Communications Federal Communications Commission Commission 445 12th Street, S.W. News Media Information (202) 418-0500 Washington, D.C. 20554 Fax-On-Demand (202) 418-2830 Internet: http://www.fcc.gov ftp.fcc.gov March 15, 2007 FCC TO HOLD OPEN COMMISSION MEETING THURSDAY, MARCH 22, 2007 The Federal Communications Commission will hold an Open Meeting on the subjects listed below on Thursday, March 22, 2007, which is scheduled to commence at 9:30 a.m. in Room TW-C305, at 445 12th Street, S.W., Washington, D.C. ITEM NO. BUREAU SUBJECT 1 MEDIA TITLE: Exclusive Service Contracts for Provision of Video Services in Multiple Dwelling Units and Other Real Estate Developments. SUMMARY: The Commission will consider a Notice of Proposed Rulemaking concerning the use of exclusive contracts for the provision of video services to multiple dwelling units (“MDUs”) or other real estate developments. 2 MEDIA TITLE: Digital Audio Broadcasting Systems and Their Impact on the Terrestrial Radio Broadcast Service (MM Docket No. 99-325). SUMMARY: The Commission will consider a Second Report and Order, First Order on Reconsideration, and Second Further Notice of Proposed Rulemaking concerning service rules and other requirements for radio stations broadcasting digital audio. *The summaries listed in this notice are intended for the use of the public attending open Commission meetings. Information not summarized may also be considered at such meetings. Consequently these summaries should not be interpreted to limit the Commission's authority to consider any relevant information. 3 MEDIA TITLE: Comparative Consideration of 76 Groups of Mutually Exclusive Applications for Permits to Construct New or Modified Noncommercial Educational (“NCE”) FM Stations. -

Public Participation Plan 2019 Update
2019 Update TABLE OF CONTENTS Glossary of Terms .....................................................................................................................................................2-4 South Carolina Department of Transportation Mission and Structure ................................................5 Introduction ..............................................................................................................................................................7 Federal Requirements ............................................................................................................................................8 Goal and Strategies.................................................................................................................................................9-10 Consultation Parties ...............................................................................................................................................10-11 The Statewide Multimodal Transportation Plan ..........................................................................................12-14 The Statewide Transportation Improvement Program ..............................................................................14-17 Evaluating the Effectiveness of Public Participation ..................................................................................17 Appendix A – Planning Process for Rural Areas of the State ...................................................................20-23 Appendix B – Consultation -
Merchandise ARTIST: Toni Braxton
Last Update: 03/17/10 ARTIST: Toni Braxton TITLE: Portrait Junior T-Shirt Black (L) Label: ANM/Atlantic Non-Music Config & Selection #: MH 516642 L Street Date: 04/20/10 Order Due Date: 04/06/10 Ship Upon Receipt - Available 4/20 UPC: 075678951534 WEBSITES: Box Count: 12 Artist Website Unit Per Set: 1 Twitter SRP: $20 Alphabetize Under: B For the latest up to date info on Merchandise OTHER SIZES: this release visit WEA.com. MH:075678951510 Portrait Junior T-Shirt Black (S)($20) MH:075678951527 Portrait Junior T-Shirt Black (M)($20) MH:075678951541 Portrait Junior T-Shirt Black (XL)($20) DESIGN ALBUM FACTS Genre: R&B Focus Markets: Atlanta, New Orleans, DC, Detroit, Houston, Baltimore, St. Louis, Miami, Las Vegas, Philadelphia, Chicago, Cleveland, Cincinnati Description: The "Portrait" design is printed on a Tultex juniors tee. The design is a graphic of her face taken from the "Yesterday" video shoot and that is her actual signature on the shirt. ARTIST & INFO Hometown: Atlanta Toni Braxton has been at the forefront of modern R&B and soul since her 1991 debut single, "Love Shoulda Brought You Home" (featured in the film, Boomerang). Her self-titled debut, which followed in 1993, proved a true pop phenomenon, earning 8x-platinum certification from the RIAA for sales in excess of 8 million. The album also saw Braxton receiving her first round of Grammy Awards, including "Best New Artist" and two "Best R&B Vocal Performance, Female" trophies, honoring the singles, "Another Sad Love Song" and "Breathe Again." "SECRETS" arrived in 1997 and indelibly confirmed Toni's status as a true international superstar.