Evaluation of Broad-Spectrum Streptomyces Sp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
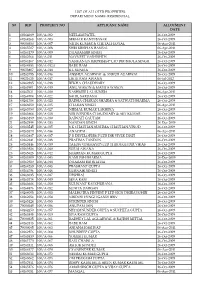
S# Rid Property No Applicant Name Allotment Date 1
LIST OF ALLOTED PROPERTIES DEPARTMENT NAME- RESIDENTIAL S# RID PROPERTY NO APPLICANT NAME ALLOTMENT DATE 1 60264689 100/A-002 NEELAM PATEL 26-Oct-2009 2 60264268 100/A-003 SEKHAR KANTI BASAK 26-Oct-2009 3 90000350 100/A-007 NITIN KUMAR & CHETALI GOYAL 06-Apr-2011 4 60265507 100/A-008 SHRI KRISHAN BANSAL 06-Apr-2011 5 60264179 100/A-009 DHARAMBIR SINGH 26-Oct-2009 6 60262564 100/A-011 NAVNEET VASHISHTH 26-Oct-2009 7 60264167 100/A-012 YASIKAN ENTERPRISES P LTD DIR BHOLA SINGH 26-Oct-2009 8 60264553 100/A-012A BABU RAM 26-Oct-2009 9 90023807 100/A-014 K L SUNEJA 26-Oct-2009 10 60265795 100/A-016 ANSHUL AGARWAL & ANKUR AGARWAL 26-Oct-2009 11 90021623 100/A-017 LIKHI RAM AWANA 06-Jul-2012 12 60262985 100/A-018 REKHA CHAUDHARY 26-Oct-2009 13 60263901 100/A-019 ANIL WASON & MAMTA WASON 26-Oct-2009 14 60267621 100/A-020 KASHMIRI LAL SUNEJA 06-Apr-2011 15 60264704 100/A-022 SAHIL SARDANA 26-Oct-2009 16 60261786 100/A-023 RADHA CHARAN SHARMA & SATWATI SHARMA 26-Oct-2009 17 60266050 100/A-025 CHARAN SINGH 06-Apr-2011 18 60263750 100/A-027 NIRMAL KUMAR LAKHINA 26-Oct-2009 19 60263868 100/A-028 VISHVENDRA CHAUDHARY & AJIT KUMAR 26-Oct-2009 20 60263189 100/A-030 RAJWATI GAUTAM 26-Oct-2009 21 60262994 100/A-033 NANDAN SINGH 26-Dec-2009 22 60263545 100/A-035 S K CHAUHAN,SUSHMA CHAUHAN,VINOD 26-Oct-2009 23 60265570 100/A-036 ANAGPAL 06-Apr-2011 24 60262887 100/A-037 P R DEVELOPERS P LTD DIR VIVEK DIXIT 26-Oct-2009 25 60262241 100/A-038 PRATIMA TANDON 26-Oct-2009 26 60263846 100/A-039 TALON VINIMAY(P) LTD THROUGH DIR.VIKAS 26-Oct-2009 27 60263926 100/A-040 -

Behsdabstractbook.Pdf
CONTENTS Messages Committees BEHSD – 2018 Organizers Detailed Scientific Program Abstracts of Plenary and Invited Lectures Abstracts of Short Oral Presentations Poster Abstracts About BRSI Sponsor Message from Professor Alok Dhawan, Director, CSIR-IITR Message from Dr D Parmar, Chairman, BEHSD-2018 Message from Professor TP Singh, President, BRSI Message from Professor Ashok Pandey, General Chair, BEHSD-2018 Bio-Innovation for Environmental and Health Sustainable Developments (BEHSD-2018) Bio-Innovation for Environmental and Health Sustainable Developments (BEHSD-2018) November 27-28, 2018 Organizing Committee Patron: Professor Alok Dhawan General Chairman: Professor Ashok Pandey Conference Chairman- Dr D Parmar Convener: Dr N Manickam Co-Conveners: Dr Kausar Ansari, Dr R Parthasarathi Treasurer- Dr K C Khulbe COA, CoFA, Sr. FAO, SPO, AO Committees and Members Fund Raising Catering and Refreshment Dr Poonam Kakkar (Chair Person) Dr K C Khulbe (Chairman) Dr Ratan Singh Ray (Convener) Dr Sanjay Yadav (Convener) Dr Sanjay Yadav Dr Sandeep Sharma Dr Sheelendra Pratap Singh Dr Ram Narayan Mr Laxmi Kant (Co-Convener) Scientific Session & Website, Publicity and Press Publication Audio visual & photography Dr K Chowdhuri (Chairman) Dr Yogeshwer Shukla (Chairman) Dr Ravi Ram Kristipati (Convener) Mr Nikhil Garg (Convener) Dr Akshay Dwarakanath Dr Akshay Dwarakanath Dr Amit Kumar Mr C M Tiwari Dr Vikas Srivatsava Mr Ali Kausar Dr R Rajagopal Mr Nasir Naqvi Dr Neeraj Satija Mr Shyam Kumar Pal Mr Sarfraj Ahmed Venue/stage Arrangement Transport Dr Alok Pandey (Chairman) Dr Ratan Singh Ray (Chairman) Dr Kausar M Ansari (Convener) Er A H Khan (Convener) Mr Yogendra Singh Dr Dhirendra Singh Mr R K Upadhyay Dr Anjeneya Dr Somendu Roy Mr Sandeep Negi Mr. -

Cicer Arietinum L.
DEVELOPMENT OF FUNCTIONAL MARKERS AND TRANSCRIPT MAP OF CHICKPEA (Cicer arietinum L.) Ms. Neha Gujaria DEVELOPMENT OF FUNCTIONAL MARKERS AND TRANSCRIPT MAP OF CHICKPEA (Cicer arietinum L.) A Thesis submitted for the degree of SUBMITTED BY Ms. Neha Gujaria CO- SUPERVISOR SUPERVISOR Dr. Rajeev K. Varshney Prof. Mangla Bhide DEPARTMENT OF ZOOLOGY SCHOOL OF BIOLOGICAL AND CHEMICAL SCIENCES DR. HARI SINGH GOUR UNIVERSITY SAGAR - 470 003 (M.P.) INDIA DECLARATION BY THE CANDIDATE (para 12b) DECLARATION I declare that the thesis entitled ―DEVELOPMENT OF FUNCTIONAL MARKERS AND TRANSCRIPT MAP OF CHICKPEA (Cicer arietinum L.)‖ is my own work conducted under the supervision of Dr. Mangla Bhide, Prof. & Head, Chairman BOS, Department of Zoology, School of Biological and Chemical Sciences, Dr. Harisingh Gour Vishwavidyalaya, Sagar (M.P.) and co- supervision of Dr. Rajeev Kumar Varshney, Director, Centre of Excellence in Genomics (CEG), International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Hyderabad (A.P.) approved by Research Degree Committee. I have put in more than 200 days of attendance with the supervisor at the centre. I further declare that to the best of my knowledge the thesis does not contain any part of work, which has been submitted for the award of any other degree either in this University or in any other Universities without proper citation. Signature of Candidate (Neha Gujaria) Signature of the Co-supervisor Signature of the Supervisor (Rajeev Kumar Varshney) (Mangla Bhide) Signature of Head of the Department CERTIFICATE OF THE SUPERVISOR (para 12 C) CERTIFICATE This is to certify that the work entitled “DEVELOPMENT OF FUNCTIONAL MARKERS AND TRANSCRIPT MAP OF CHICKPEA (Cicer arietinum L.)” is a piece of research work done by Ms. -

No. 19 & 20 October
ISSN 0409-7467 CSIR News NEWSLETTER OF THE COUncIL OF SCIENTIFIC & INDUSTRIAL RESEARCH Volume 65 Nos. 19 & 20 website: http://www.csir.res.in October 2015 In The News In This Issue CSIR Celebrates its 73rd Foundation Day 217In The News • CSIR Celebrates its 73rd Foundation Day • CSIR Announces Awards for Scientists 232Workshops & Symposia • CSIR-NISCAIR Organises Workshop on Training Journalists in S&T Reporting • National Symposium on Acoustics held at CSIR-NIO 238Honours & Awards On the dais during the 73rd CSIR Foundation Day Function at Vigyan Bhawan, New Delhi 240Appointments (from left): Dr. Girish Sahni, Director General of CSIR, Mr Y.S. Chowdary, Minister of State for Science and Technology and Earth Sciences, Dr. Harsh Vardhan, Union Minister for Science & Technology and Earth Sciences and Mr A.S. Kiran Kumar, Secretary, Department of Space and Chairman, ISRO THE Union Minister of Science problems of the country. Speaking at & Technology and Earth Sciences and the CSIR Foundation Day Celebrations, Vice President, Council of Scientific the Minister said that he was proud of and Industrial Research CSIR, the diverse research taking place in the Dr. Harsh Vardhan, has called for labs from aeroplanes to nanomaterials, mobilizing the entire potential of CSIR from ayurgenomics to new drugs and in a structured manner to solve various petroleum. The Council of Scientific & Dr. Harsh Vardhan said that while In The News Industrial Research (CSIR) celebrated its everyone was involved in the country’s 73rd Foundation Day on 26 September freedom struggle in 1942, for someone 2015. The function was held in the to even think of giving birth to an entity Vigyan Bhawan in New Delhi. -

Annual Report 2016-2017 ISCA Annual Report 2016-2017 ANNUAL REPORT 2016-2017
ISCA Annual Report 2016-2017 ISCA Annual Report 2016-2017 ANNUAL REPORT 2016-2017 THE INDIAN SCIENCE CONGRESS ASSOCIATION 14, DR. BIRESH GUHA STREET, KOLKATA - 700 017 ISCA Annual Report 2016-2017 Published by: The Indian Science Congress Association 14, Dr. Biresh Guha Street, Kolkata - 700 017 Printed at: M/s. Independent Printers One, Park Side Road, Kolkata – 700 026 ISCA Annual Report 2016-2017 CONTENTS PREFACE Page PROFILE OF THE INDIAN SCIENCE CONGRESS ASSOCIATION 1-2 THE HUNDREDTH FOUR SESSION OF INDIAN SCIENCE CONGRESS 3 Inaugural Session 3 Children Science Congress 3 Women’s Science Congress 4 Science Communicators’ Meet (SCM) 4 Science Exhibition: Pride of India Expo- 104th ISC 6 Valedictory Session 6 Technical Programmes 7 Activities in Sections 25 ISCA Endowment Awards/Lectures 25 Major Recommendations 27 OTHER ACTIVITIES ISCA Chapters 35-41 Celebration of Hindi Programme 42 Publications 42 OTHER ITEMS ISCA Meetings 42 ISCA Representation in other Organizations 43 Membership 44 Organizational Set-up 44 ISCA Annual Report 2016-2017 ACKNOWLEDGEMENTS Page ANNEXURE -I 47 Title of Addresses of Sectional Presidents of 104th ISC Session ANNEXURE -II 48 Platinum Jubilee Lectures of 104th ISC Session ANNEXURE -III 49 Titles of Symposia on specialized topics organised by the Sections of 104th Indian Science Congress ANNEXURE -IV 50 List of Young Scientist Awardees for the year 2016-2017 ANNEXURE -V 52 List of Best Poster Presentation Awardees for 2016-2017 ANNEXURE -VI 54 List of infosys Foundation - ISCA Travel Awardees for 2016-2017 APPENDIX -I 55 Members of the Council for 2016-2017 APPENDIX -II 56 Members of the Council for 2017-2018 APPENDIX -III 57 Personnel APPENDIX -IV 58 General Presidents of ISCA APPENDIX -V 62 General Secretaries of ISCA APPENDIX -VI 63 Treasurers of ISCA AUDIT REPORT & ACCOUNTS 67-97 ISCA Annual Report 2016-2017 PREFACE March 31, 2017 marked the completion of the 104th year of The Indian Science Congress Association. -

Eligible Voter's List
Sl.No ASI ID Name State 1 10 BHAT K L DELHI 2 13 ADINARAYANA RAO S V ANDHRA PRADESH 3 20 AGARWAL S K WEST BENGAL 4 22 AGARWAL Y M HARYANA 5 26 AGARWAL P K JHARKHAND 6 32 AHMAD ABDUL HAI BIHAR 7 33 AHUJA A M LT GENERAL DELHI 8 35 SINGHVI A M RAJASTHAN 9 39 ALMAST S C DELHI 10 46 AMARESH BHASKAR NALLAVANDU AIR MSHL KARNATAKA 11 47 AMARESWAR T KARNATAKA 12 48 AMARJIT SINGH PUNJAB 13 49 AMBIKE V S MAHARASTRA 14 55 ANAND PRAKASH UTTAR PRADESH 15 58 ANANTHAKRISHNAN L TAMIL NADU 16 59 NARAYAN PRASAD K TAMILNADU 17 60 ANANTHANARAYANA RAO N KARNATAKA 18 72 ANTIA N H MAHARASTRA 19 76 APTE B P MAHARASTRA 20 79 ARCANJO DE MENESES GOA 21 83 ARORA S MAHARASTRA 22 91 ASOPA H S UTTAR PRADESH 23 95 VIKRAM PRATAP SINGH LT. COL. DELHI 24 101 BAJAJ P S BIHAR 25 108 BAPAT V C MADHYA PRADESH 26 109 BHATTACHARYYA S MAHARASTRA 27 118 KRISHNA HANDE H TAMIL NADU 28 123 BALLAL C R KARNATAKA 29 127 BAMRAH N S BRIG CHANDIGARH 30 130 BANERJEE L K DELHI 31 131 BANERJEE S WEST BENGAL 32 134 ASHIM BANERJEE(COL) WEST BENGAL 33 138 BAPAT S D MAHARASHTRA 34 140 DEEPAK V BHATT GUJARAT 35 147 PRANAB KUMAR BASU UTTAR PRADESH 36 149 BAWA H S PUNJAB 37 153 BEHERA D K ODISHA 38 159 BHAJEKAR A B MAHARASTRA 39 161 BAKTHAVATSALAM G TAMIL NADU 40 163 BHALERAO R A MAHARASHTRA 41 170 BHANUSHALI H S MAHARASTRA 42 176 BHARUCHA P B MAHARASTRA 43 179 BHASALE S P MAHARASHTRA 44 184 BHATT M V MAHARASHTRA 45 188 BHATHENA T R MAHARASHTRA 46 189 JAGDISHWER BHATT RAJASTHAN 47 195 SINGARAJU KRISHNA PRABHAKAR TELANGANA 48 199 RAVIKANTH C BANGALORE KARNATAKA 49 200 BHAKTA V P MAHARASTRA 50 206 BIMA -

Sr. Roll No. Name of Advocate Father's/Husband's Name
Roll No. Enrollment Date of Sr. Name of Advocate Complete Address Telephone No. Email Address No./Year/Council Enrollment Father's/Husband's Name ALJ/0001/2020 [email protected] H- 15/F Gyan sarovar Colony, 1. Rajendra Prasad UP02351/84 15/07/1984 9897508384 Ramghat Road, aligarh Chimman Lal ALJ/0002/2020 - UP04195/04 2. Kunwar istkar Ali 17/07/2007 Vill & Post- Rajpur, Aligarh 9411655376 Iliyas Ali ALJ/0003/2020 [email protected] UP01158/07 H.N. 19/24 Krishnapuri, Khirni 3. Ullas Saraswat 10/02/2007 9012273337 gate, Aligarh Kedar Nath Saraswat ALJ/0004/2020 - 4. Mahendra Kumar Kulshrestha UP00295/81 21/01/19981 15/67 Tamoli Para, Aligarh 8534965398 Prakash Chandra Kulshrestha ALJ/0005/2020 - UP02295/99 Ladiya ki chowki, School No. 4 5. Jagdish prashad Rathor 28/03/1999 7599015729 jayganj, Aligarh Sohan pal rathor ALJ/0006/2020 2/107A, pachori nagar near - 9411488406 6. Rajesh kumar Sharma UP11172/99 17/06/1999 S.G.D public school Begam bag, Devi prashad Sharma koil, Aligarh ALJ/0007/2020 - UP04222/03 2/134, pachori nagar 7. Hutashan Kumar 12/04/2003 8307805503 Chandaniya chok, Aligarh Darshan Singh ALJ/0008/2020 - 8. Rakesh Chandra UP00670/98 23/02/1998 2/275 New Begam Bag Aligarh 9412389333 Ramesh chandra Verma ALJ/0009/2020 5/91 beema nagar soot mill, G.T. - UP02997/89 9. Ram Baboo 14/05/1989 Road, in front of TVS show 8006077719 Ram chandra room Koil Aligarh 10. ALJ/0010/2020 UP00925/13 18/02/2013 Shanti puram, Agra road aligarh 9412625250 - Chandresh Upadhyay 1 Roll No. -

Proceedings of the Regional Consultation on the Promotion of Pulses in Asia for Multiple Health Benefits
viii VII. Country status reports RAP Publication 2015/05 Proceedings of the regional consultation on the promotion of pulses in Asia for multiple health benefits Bangkok, Thailand, 29 to 30 June 2015 Compiled and edited by Subash Dasgupta and Indrajit Roy FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS REGIONAL OFFICE FOR ASIA AND THE PACIFIC Bangkok, 2016 i Proceedings of the regional consultation on the promotion of pulses in Asia for multiple health benefits The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. ISBN 978-92-5-109079-4 © FAO, 2016 FAO encourages the use, reproduction and dissemination of material in this information product. Except where otherwise indicated, material may be copied, downloaded and printed for private study, research and teaching purposes, or for use in non-commercial products or services, provided that appropriate acknowledgement of FAO as the source and copyright holder is given and that FAO’s endorsement of users’ views, products or services is not implied in any way. -

The Year Book 2020
THE YEAR BOOK 2020 INDIAN ACADEMY OF SCIENCES Bengaluru Postal Address: Indian Academy of Sciences Post Box No. 8005 C.V. Raman Avenue Sadashivanagar Post, Raman Research Institute Campus Bengaluru 560 080 India Telephone : +91-80-2266 1200, +91-80-2266 1203 Fax : +91-80-23616094 Email : [email protected], [email protected] Website : www.ias.ac.in © 2020 Indian Academy of Sciences Information in this Year Book is updated up to 31 January 2020. Editorial & Production Team: Nalini, B.R. Thirumalai, N. Vanitha, M. Published by: Executive Secretary, Indian Academy of Sciences Text formatted by WINTECS Typesetters, Bengaluru (Ph. +91-80-2332 7311) Printed by The Print Point, Bengaluru CONTENTS Page Section A: Indian Academy of Sciences Activities – a profile ................................................................. 2 Council for the period 2019–2021 ............................................ 6 Office Bearers ......................................................................... 7 Former Presidents ................................................................... 8 The Academy Trust ................................................................. 9 Section B: Professorships Raman Chair ........................................................................... 12 Jubilee Chair ........................................................................... 15 Janaki Ammal Chair ................................................................ 16 The Academy–Springer Nature Chair ...................................... 16 Section C: -

Member Number Title Name State 10 Dr Khanaya Kal Bhat Delhi 13 Dr
Member Number Title Name State 10 Dr Khanaya Kal Bhat Delhi 13 Dr S V Adinarayana Andhra Pradesh 22 Dr AGARWAL Y M Haryana 32 Dr Ahmad Abdul Hai Bihar 35 Dr Ajeet Mal Singhvi Rajasthan 46 Dr Nallavandu Bhaskar Amaresh Karnataka 49 Dr Vilas Shankar Ambike Maharashtra 58 Dr L Ananthakrishnan Tamil Nadu 79 Dr Arcanjo Menezes Goa 91 Dr H. S. Asopa Uttar Pradesh 118 Dr Krishna Hande H Tamil Nadu 123 Dr C R Ballal Karnataka 131 Dr Sachinandan Banerjee West Bengal 138 Dr Sharad D. Bapat Maharashtra 161 Dr Bakthavathsalam Tamil Nadu 170 Dr H S Bhanushali Maharashtra 189 Dr Jagdishwer Bhatt Rajasthan 195 Dr Singaraju Krishna Prabhakar Telangana 199 Dr RAVIKANTH C BANGALORE Karnataka 200 Dr Bhakta V P Maharashtra 210 Dr R K Bisarya Madhya Pradesh 216 Dr Prahlad Kumar Bilwani Gujarat 218 Dr Vinaya Chandra Bothra Rajasthan 235 Dr Amalendu Chakraborty West Bengal 241 Dr P V Chalapathi Rao Telangana 262 Dr SUBIR K CHATTERJEE West Bengal 290 Dr P P Chawla Jharkhand 307 Dr D.K. Choudhary Bihar 316 Dr Subhash J Dalal Maharashtra 317 Dr Dulal Chandra Dalui West Bengal 338 Dr Govind V. Datar Maharashtra 341 Dr DESARKAR B K Chhattisgarh 373 Dr DESHMUKH S S Maharashtra 376 Dr THUSOO T K Haryana 377 Dr Suresh Gajanan Deshpande Maharashtra 382 dr Devdas Hegde Karnataka 391 Dr Indar Kumar Dhawan Haryana 397 Dr Dilip P Shah Gujarat 405 Dr DORAIRAJAN T Tamil Nadu 429 Dr K.Rajasekaran Tamil Nadu 440 Dr Nripendra Nath Samanta West Bengal 448 Dr Durga Dutt Gaur Maharashtra 457 Dr Devraj L ghevariya Gujarat 468 Dr D K Gupta Uttar Pradesh 478 Dr Gobinda ram Manna West Bengal 483 Dr Satish K. -

Honorary Fellows
HONORARY FELLOWS Year of Name, Designation and Address Election 2010 AMBANI, Mukesh (b. 1957), Chairman & Managing Director, Reliance Industries Limited, Maker Chambers - IV, 222, Nariman Point, Mumbai - 400021; Tel. (022) 22785000(O); Email : [email protected] 2013 BAMJI, Mahtab S. (b. 1934) Ph.D., FNAAS, FAMS, FNA, INSA Emeritus Scientist, Dangoria Charitable Trust, Hyderabad - 500020; Res. 211, Sri Dattasai Apartments, RTC Cross Rds, Hyderabad - 500020; Tel. (040) 27615148(R), 09246886442, 09505838921(M); Email : [email protected]; mahtabbamji@ yahoo.com; Sp. Biochemistry/Nutrition. 1980 HARRISON, J.D.L. Energy Adviser, Overseas Development Administration, London; 2000 JOSHI, Murli Manohar (b. 1934) M.Sc., D.Phil., Member of Parliament (Lok Sabha) and formerly Cabinet Minister for Home Affairs, HRD; S&T and DOD, Union Govt. of India, New Delhi; Formerly Professor & Head, Department of Physics, University of Allahabad; Res. 'Angiras' 9/10-A, Tagore Nagar, Anukul Chandra Banerjee Road, Allahabad - 211002; and 6, Raisina Road, New Delhi - 110001; Tel. (0532) 2465008, 2466860 (Alld.)(R); (011) 23718444, 23711144 (N.Delhi)(R); Fax : 23711772 (N.Delhi); Email : murli@sansad. nic.in; Sp. Spectroscopy. 1980 KRANZ, Jurgen (b. 1925) Ph.D., Formerly Professor, Dept. of Phytopathology & Appl. Entomology, Justus-Liebig-Universitat; Res. 35394 Giessen, F.R. Germany, Rehschneise 75; Tel. (0641) 492836; Fax : 43875(R); Sp. Epidemiology of Plant Diseases/ Tropical Plant Diseases. 1978 PRAMER, David (b. 1923) Ph.D.(Rutgers), Professor of Microbiology & Associate Vice President, Rutgers University, Office of Corporate Liaison, 377 Hoes Lane, Piscataway, New Jersey, USA 08855; Res. 208 Hampshire Court, Piscataway, New Jersey, USA 08854; Tel. 908-445-2863(O), 908-463- 3866(R);Sp. -

The Extent of Grain Yield and Plant Growth Enhancement by Plant Growth-Promoting Broad-Spectrum Streptomyces Sp
Gopalakrishnan et al. SpringerPlus (2015) 4:31 DOI 10.1186/s40064-015-0811-3 a SpringerOpen Journal RESEARCH Open Access The extent of grain yield and plant growth enhancement by plant growth-promoting broad-spectrum Streptomyces sp. in chickpea Subramaniam Gopalakrishnan*, Vadlamudi Srinivas, Gottumukkala Alekhya, Bandikinda Prakash, Himabindu Kudapa, Abhishek Rathore and Rajeev Kumar Varshney Abstract The physiological and molecular responses of five strains of Streptomyces sp. (CAI-17, CAI-68, CAI-78, KAI-26 and KAI-27), with their proven potential for charcoal rot disease control in sorghum and plant growth-promotion (PGP) in sorghum and rice, were studied to understand the mechanisms causing the beneficial effects. In this investigation, those five strains were evaluated for their PGP capabilities in chickpea in the 2012–13 and 2013–14 post-rainy seasons. All of the Streptomyces sp. strains exhibited enhanced nodule number, nodule weight, root weight and shoot weight at 30 days after sowing (DAS) and pod number, pod weight, leaf area, leaf weight and stem weight at 60 DAS in both seasons over the un-inoculated control. At crop maturity, the Streptomyces strains had enhanced stover yield, grain yield, total dry matter and seed number plant−1 in both seasons over the un-inoculated control. In the rhizosphere, the Streptomyces sp. also significantly enhanced microbial biomass carbon, dehydrogenase activity, total nitrogen, available phosphorous and organic carbon in both seasons over the un-inoculated control. Of the five strains of Streptomyces sp., CAI-17, CAI-68 and CAI-78 were superior to KAI-26 and KAI-27 in terms of their effects on root and shoot development, nodule formation and crop productivity.