Ore Types of the Auriferous Lalor VMS Deposit, Snow Lake, Manitoba: Implications for Genesis and Post Depositional Processes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Manitoba Regional Health Authority (RHA) DISTRICTS MCHP Area Definitions for the Period 2002 to 2012
Manitoba Regional Health Authority (RHA) DISTRICTS MCHP Area Definitions for the period 2002 to 2012 The following list identifies the RHAs and RHA Districts in Manitoba between the period 2002 and 2012. The 11 RHAs are listed using major headings with numbers and include the MCHP - Manitoba Health codes that identify them. RHA Districts are listed under the RHA heading and include the Municipal codes that identify them. Changes / modifications to these definitions and the use of postal codes in definitions are noted where relevant. 1. CENTRAL (A - 40) Note: In the fall of 2002, Central changed their districts, going from 8 to 9 districts. The changes are noted below, beside the appropriate district area. Seven Regions (A1S) (* 2002 changed code from A8 to A1S *) '063' - Lakeview RM '166' - Westbourne RM '167' - Gladstone Town '206' - Alonsa RM 'A18' - Sandy Bay FN Cartier/SFX (A1C) (* 2002 changed name from MacDonald/Cartier, and code from A4 to A1C *) '021' - Cartier RM '321' - Headingley RM '127' - St. Francois Xavier RM Portage (A1P) (* 2002 changed code from A7 to A1P *) '090' - Macgregor Village '089' - North Norfolk RM (* 2002 added area from Seven Regions district *) '098' - Portage La Prairie RM '099' - Portage La Prairie City 'A33' - Dakota Tipi FN 'A05' - Dakota Plains FN 'A04' - Long Plain FN Carman (A2C) (* 2002 changed code from A2 to A2C *) '034' - Carman Town '033' - Dufferin RM '053' - Grey RM '112' - Roland RM '195' - St. Claude Village '158' - Thompson RM 1 Manitoba Regional Health Authority (RHA) DISTRICTS MCHP Area -

The Arctic Gateway Group Is Owned by First Nations and Bayline Communities, Fairfax and Agt Foods, Building a Natural Resources
THE ARCTIC GATEWAY GROUP IS OWNED BY FIRST NATIONS AND BAYLINE COMMUNITIES, FAIRFAX AND AGT FOODS, BUILDING A NATURAL RESOURCES GATEWAY THROUGH THE ARCTIC TO THE WORLD. Arctic Gateway Group LP Arctic_Gateway ArcticGateway 728 Bignell Ave. ArcticGateway The Pas, MB R9A 1L8 1-888-445-1112 [email protected] www.arcticgateway.com ABOUT THE GATEWAY The Arctic Gateway Group LP owns and operates the Port of Churchill, Canada’s only Arctic seaport serviced by rail, on the Hudson Bay Railway, running from The Pas to Churchill, Manitoba. Strategically located on the west coast of Hudson Bay, the Arctic Gateway is the front door to Western Canada, linking Canadian trade in resources to the global marketplace. The Arctic Gateway’s logistical advantage, rail assets and unique location provide direct and efficient routes to markets for Canada’s abundant natural resources and manufactured products, while connecting Canadian consumers and importers to the world marketplace via the North. Hudson Bay Railway (CN, KRC) port of The Hudson Bay Railway is made up of 627 miles port location interchange churchill hudson bay railroad (hbr) agg HBR operating of former Canadian National (CN) trackage, with a agg railroad agreement network that connects with CN in The Pas, running north through Manitoba to the Hudson Bay at the lynn lake kelsey gillam Port of Churchill. The Hudson Bay Railway is a vital transportation pukatawagan thompson link in northern Manitoba, hauling perishables, automobiles, frac ilford sherridon thicket Flin Flon sand, construction material, heavy and dimensional equipment, sherritt jct wabowden scrap, hazardous materials, kraft paper, concentrates, containers, Cranberry portage the pas the pas jct fertilizer, wheat and other grain products. -

Errata Since Publication in June 2010, We Have Discovered an Number of Items That Needed Either Clarity Or Correction
Metis Health Status and Healthcare Use in Manitoba Errata Since publication in June 2010, we have discovered an number of items that needed either clarity or correction. Updated pages are # 10-11, 56, 141-142, 234-235, 254, 272, 286, 299-301, 456, 460, 480 &582. All the updated pages are attached. Manitoba Centre for Health Policy Errata_Nov_2012.indd 1 28/11/2012 2:04:52 PM Chapter 1: Introduction and Methods This page edited September 23, 2010. Figure 1.4: Villages, Towns, Cities, or Unorganized Territories Where Metis Live in Manitoba, 2009 Where Metis Live in Manitoba, 2009 Villages, towns, cities, or unorganized territories See next page for Metis 1 community locations code key 2 3 6 14 5 4 7 8 11 10 19 15 20 9 21 22 12 16 18 23 24 13 17 2825 27 26 29 30 31 37 32 3433 35 38 36 39 119 42 40 41 43 44 4950 65 51 45 66 58 67 52 68 72 4647 53 69 55 70 54 71 48 73 120 56 57 75 121 62 74 59 76 77 80 60 64 78 79 104 61 63 81 123122 105 82 83 109 124 126 106 84 85 86 108 125 107 89 88 94 90 93 127 110 116 91 87 11792 103 95 98 102 111 96 100 101 115 118 99 97 139 128 112 129 130 132 133 134 113 136 131 135 114 137 138 Source: MCHP/MMF, 2010 10 | University of Manitoba Metis Health Status and Healthcare Use in Manitoba This page edited September 23, 2010. -

Directory – Indigenous Organizations in Manitoba
Indigenous Organizations in Manitoba A directory of groups and programs organized by or for First Nations, Inuit and Metis people Community Development Corporation Manual I 1 INDIGENOUS ORGANIZATIONS IN MANITOBA A Directory of Groups and Programs Organized by or for First Nations, Inuit and Metis People Compiled, edited and printed by Indigenous Inclusion Directorate Manitoba Education and Training and Indigenous Relations Manitoba Indigenous and Municipal Relations ________________________________________________________________ INTRODUCTION The directory of Indigenous organizations is designed as a useful reference and resource book to help people locate appropriate organizations and services. The directory also serves as a means of improving communications among people. The idea for the directory arose from the desire to make information about Indigenous organizations more available to the public. This directory was first published in 1975 and has grown from 16 pages in the first edition to more than 100 pages in the current edition. The directory reflects the vitality and diversity of Indigenous cultural traditions, organizations, and enterprises. The editorial committee has made every effort to present accurate and up-to-date listings, with fax numbers, email addresses and websites included whenever possible. If you see any errors or omissions, or if you have updated information on any of the programs and services included in this directory, please call, fax or write to the Indigenous Relations, using the contact information on the -

Pdfs GST-HST Municipal Rebates 2019 E Not Finished.Xlsx
GST/HST Incremental Federal Rebate for Municipalities Report - January 1 to December 31, 2019 Manitoba PAYMENT LEGAL NAME CITY NAME FSA AMOUNT 2625360 MANITOBA ASSOCIATION INC. NEEPAWA R0J $2,993.73 285 PEMBINA INC WINNIPEG R2K $10,624.47 4508841 MANITOBA ASSOCIATION INC WINNIPEG R2K $517.02 474 HARGRAVE CORPORATION WINNIPEG R3A $2,504.76 6869166 MANITOBA LTD. SANFORD R0G $7,370.38 ACADEMY ROAD BUSINESS IMPROVMENT ZONE WINNIPEG R3N $1,389.15 AGASSIZ WEED CONTROL DISTRICT BEAUSEJOUR R0E $549.30 ALTONA RURAL WATER SERVICES CO-OP LTD ALTONA R0G $1,860.62 ARBORG BI-FROST PARKS & RECREATION COMMISSION ARBORG R0C $5,326.89 ARGYLE-LORNE-SOMERSET WEED CONTROL DISTRICT BALDUR R0K $553.10 ARLINGTONHAUS INC. WINNIPEG R2K $11,254.49 ARTEMIS HOUSING CO-OP LTD WINNIPEG R3A $2,784.09 ASTRA NON-PROFIT HOUSING CORPORATION WINNIPEG R2K $2,993.66 AUTUMN HOUSE INC. WINNIPEG R3E $3,532.89 B&G UTILITIES LTD BRANDON R7B $3,643.38 BAPTIST MISSION APARTMENTS INC. WINNIPEG R3E $2,224.34 BARROWS COMMUNITY COUNCIL BARROWS R0L $3,837.41 BEAUSEJOUR BROKENHEAD DEVELOPMENT CORP BEAUSEJOUR R0E $3,583.19 BETHANIAHAUS INC. WINNIPEG R2K $17,881.45 BIBLIOTHÉQUE MONTCALM LIBRARY SAINT-JEAN-BAPTISTE R0G $180.01 BIBLIOTHÉQUE REGIONALE JOLYS REGIONAL LIBRARY SAINT-PIERRE-JOLYS R0A $267.88 BIBLIOTHÉQUE TACHÉ LIBRARY LORETTE R0A $851.71 BISSETT COMMUNITY COUNCIL BISSETT R0E $2,919.53 BLUMENFELD HOCHFELD WATER CO-OP LTD WINKLER R6W $770.13 BLUMENORT SENIOR CITIZENS HOUSING INC. STEINBACH R5G $515.67 BOISSEVAIN - MORTON LIBRARY AND ARCHVIES BOISSEVAIN R0K $784.80 BOISSEVAIN AND MORTON -
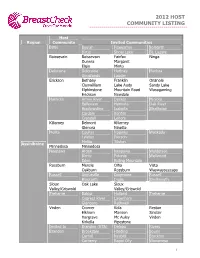
2012 Host Community Listing
2012 HOST COMMUNITY LISTING Host Region Community Invited Communities Birtle Beulah Foxwarren Solsgirth Birtle Shoal Lake St. Lazare Boissevain Boissevain Fairfax Ninga Dunrea Margaret Elgin Minto Deloraine Deloraine Hartney Medora Goodlands Lauder Erickson Bethany Franklin Onanole Clanwilliam Lake Audy Sandy Lake Elphinstone Mountain Road Wasagaming Erickson Newdale Hamiota Arrow River Decker Miniota Belleview Hamiota Oak River Bradwardine Isabella Strathclair Cardale Kenton Crandall Lenore Killarney Belmont Killarney Glenora Ninette Melita Coulter Napinka Waskada Lyleton Pierson Melita Tilston Assiniboine Minnedosa Minnedosa Neepawa Arden Neepawa Waldersee Birnie Polonia Wellwood Eden Riding Mountain Rossburn Menzie Olha Vista Oakburn Rossburn Waywayseecapo Russell Angusville Dropmore Russell Binscarth Inglis Shellmouth Sioux Oak Lake Sioux Valley/Griswold Valley/Griswold Treherne Baldur Holland Treherne Cypress River Lavenham Glenboro Rathwell Virden Cromer Kola Reston Elkhorn Manson Sinclair Hargrave Mc Auley Virden Kirkella Pipestone Invited to Brandon (R7A) Deleau Rivers Brandon Brookdale Harding Souris Carroll Nesbitt Stockton Carberry Rapid City Wawanesa 1 2012 HOST COMMUNITY LISTING Host Region Community Invited Communities Invited to Cartwright Holmfield Mather Assiniboine Crystal City (cont.) Invited to Glenella Kelwood McCreary Churchill Churchill Cross Lake Cross Lake Gillam Gillam Ilford Shamattawa Leaf Rapids Leaf Rapids South Indian Lake Lynn Lake Brochet Lynn Lake Lac Brochet Tadoule Lake Burntwood Nelson House Nelson -

Population Report June 1, 2016
Manitoba Population Report June 1, 2016 Manitoba Health, Seniors and Active Living Manitoba Health, Healthy Living and Seniors Population Report June 1, 2016 Inquiries can be directed to: Manitoba Health, Healthy Living and Seniors Health Information Management Branch Room 4040 300 Carlton Street Winnipeg Manitoba R3B 3M9 Telephone: (204) 788-6354 2016 Population Publication Report - Contents 1 Introduction Foreword Population Changes June 1, 2015 to June 1, 2016 Deaths by Age (Fig. 1a) Deaths by Age and Gender (Fig. 1b and 1c) Migration In and Out of the Province by Age (Fig. 2a) Migration In and Out of the Province by Age and Gender (Fig. 2b and 2c) 2 Population of Manitoba – June 1, 2016 Population of Manitoba by Age and Gender Population of Manitoba by Age (Fig. 3a) Population of Manitoba by Age and Gender (Fig. 3b and 3c) Map of Manitoba RHA Boundaries (Fig. 4) Population of Manitoba by RHA, Age, and Gender Population of Manitoba First Nations, by RHA, Age and On/Off Reserve Status Population of Manitoba by RHA, 2012 – 2016 Percent of Population by RHA, 2012 and 2016 (Fig. 5) Population of Manitoba by RHA (Age 65+), 2012 – 2016 Percent of Population by RHA (Age 65+), 2012 – 2016 (Fig. 6) 3 Population of Regional Health Authorities – June 1, 2016 Interlake-Eastern RHA Population by District, Age, and Gender Population by District, 2012 – 2016 Population by District (Age 65+), 2012 – 2016 Population by Municipality, Age, and Gender Population by Municipality, 2012 – 2016 Population by Municipality (Age 65+), 2012 – 2016 Northern RHA -

Summerberry Marsh Trappers - Other Communities Current As of August 12, 2016
Summerberry Marsh Trappers - Other Communities Current as of August 12, 2016 A Adams, Joshua G. Mafeking Albert, Jacob Norway House Alguire, Victor Mafeking Amer, Victor Cranberry Portage Anderson, Barney Cranberry Portage Anderson, Sam Cranberry Portage Aoust, Paul D Norway House Arthurson, Arthur Norway House Arthurson, Benjamin Norway House Arthurson, John Norway House Asmus, Charles Cranberry Portage Asmus, Gamet Cranberry Portage Asmus, Walter Cranberry Portage Asmus, Willard Jr. Cranberry Portage Asuze, W.G. Misc - Wabowden Atchinson, N.R Bellsite Atkinson, Frank St Marks Atwater, John G. Mafeking Azure, Moise Misc- Mile 122 HBRlwy Azure, William Misc - Mile 122 HBRlwy B Bacon, Ernest St Eustace Bacon, Louis Winnipeg Baginski, Joe Misc - Victoria BC Bailey, Edmund Norway House Baker, Billy Cranberry Portage Baker, Durlin Cranberry Portage Baker, Norman Cranberry Portage Barber, Pete Cranberry Portage Barker, Albert Barrows and Baden Barker, Alfred Barrows and Baden Barker, Fred Barrows and Baden Barker, Vern Barrows and Baden Barlage, August Wanless Barron, Aime Mafeking Beardy, Sandy Cross Lake Bennett, R.T. Bellsite Berg, Rueben Cranberry Portage Bickle, Nelson Mafeking Bjornson, Paul Misc - Thicket Portage Blacksmith, Henry Cross Lake Blacksmith, John Cross Lake Bousquest., L.M. Gladstone Bradbury, Bill Mafeking Bradbury, George Mafeking Bradbury, Ted Mafeking Bradbury, Don Birch River Brooks, W. S. Cranberry Portage Bums, William Cranberry Portage Bun, R.L. Misc - Gillam Burdeny, Adam Ethelbert Burdeny, Paul Ethelbert Burke, Donald Cranberry Portage C Calcutt, Bert Herb Lake Wekusko Campbell, Jack Mafeking Campbell, James Winnipegosis Campbell, Robert Sherridon Kississing Campbell, William M.D. Norway House Captain, Henry G. Norway House Chartand, Bruce St. Ambroise Chartarand, Baptiste St Laurent Chartrand, Emile St. -

Release, Transport and Attenuation of Metals from an Old Tailings Impoundment
Applied Geochemistry Applied Geochemistry 20 (2005) 639–659 www.elsevier.com/locate/apgeochem Release, transport and attenuation of metals from an old tailings impoundment M.C. Moncur a, C.J. Ptacek a,b,*, D.W. Blowes a, J.L. Jambor c a Department of Earth Sciences, University of Waterloo, 200 University Avenue, Waterloo, Ont., Canada N2L 3G1 b National Water Research Institute, Environment Canada, 867 Lakeshore Road, P.O. Box 5050, Burlington, Ont., Canada L7R 4A6 c Department of Earth and Ocean Sciences, University of British Columbia, 6339 Stores Road, Vancouver, BC, Canada V6T 1Z4 Received 24 May 2004; accepted 25 September 2004 Editorial handling by R. Fuge Abstract The oxidation of sulfide minerals from mine wastes results in the release of oxidation products to groundwater and surface water. The abandoned high-sulfide Camp tailings impoundment at Sherridon, Manitoba, wherein the tailings have undergone oxidation for more than 70 a, was investigated by hydrogeological, geochemical, and mineralogical techniques. Mineralogical analysis indicates that the unoxidized tailings contain nearly equal proportions of pyrite and pyrrhotite, which make up to 60 wt% of the total tailings, and which are accompanied by minor amounts of chal- copyrite and sphalerite, and minute amounts of galena and arsenopyrite. Extensive oxidation in the upper 50 cm of the tailings has resulted in extremely high concentrations of dissolved SO4 and metals and As in the tailings pore water À1 À1 À1 À1 À1 À1 (pH < 1, 129,000 mgL Fe, 280,000 mgL SO4, 55,000 mgL Zn, 7200 mgL Al, 1600 mgL Cu, 260 mgL Mn, 110 mgLÀ1 Co, 97 mgLÀ1 Cd, 40 mgLÀ1 As, 15 mgLÀ1 Ni, 8 mgLÀ1 Pb, and 3 mgLÀ1 Cr). -

Vendor Payments Over $50,000 20
FOR THE YEAR ENDED MARCH 31, 2020 Vendor Payments Over $50,000 20 VENDOR STATEMENT OF CONSOLIDATED FUND PAYMENTS IN EXCESS OF $50,000 TO CORPORATIONS, FIRMS, INDIVIDUALS, OTHER GOVERNMENTS AND GOVERNMENT AGENCIES For the fiscal year ended March 31, 2020 (UNAUDITED) ………………………………………………… INFORMATION 1. This statement lists, in alphabetical order within Department or Special Operating Agency, each payee whose total payments by that individual department or special operating agency exceeded $50,000 during the fiscal year. Payments are presented on a gross basis to show all amounts which were charged to the 2019-2020 fiscal period for the purchase of goods and services, investment and loan transactions, grant payments and refunds as well as payments from amounts held in trust on behalf of others. 2. Where a Department or Special Operating Agency did not have any payee meeting the threshold of total payments exceeding $50,000, it is noted that there are “NO REPORTING VENDORS”. 3. Where multiple, same name vendors are found within a city/town, they are applicable to different locations. 4. Where the name of a city/town appears twice, in succession, the first is attached to the vendor’s name, the second is applicable to the vendor’s address. 5. This statement also lists, in alphabetical order within department, vendors from whom departmental purchases, using the National Bank of Canada procurement cards, are equal to or exceeding $25,000 during the fiscal year. 6. Payments in other currencies are shown in the Canadian dollar equivalent at the time of payment. 7. The location of the payee is shown except where payment was made to an address in Winnipeg. -

Pdfs GST-HST Municipal Rebates 2018 E.Xlsx
GST/HST Incremental Federal Rebate for Municipalities Report - January 1 to December 31, 2018 Manitoba PAYMENT LEGAL NAME CITY NAME FSA AMOUNT 2625360 MANITOBA ASSOCIATION INC. NEEPAWA R0J $1,112.09 285 PEMBINA INC WINNIPEG R2K $11,916.31 4508841 MANITOBA ASSOCIATION INC WINNIPEG R2K $1,519.18 474 HARGRAVE CORPORATION WINNIPEG R3A $2,653.46 6869166 MANITOBA LTD. SANFORD R0G $6,712.69 7049651 MANITOBA ASSOCIATION INC. WINNIPEG R3B $330,332.94 ACADEMY ROAD BUSINESS IMPROVMENT ZONE WINNIPEG R3N $6,535.53 AGASSIZ WEED CONTROL DISTRICT BEAUSEJOUR R0E $244.81 ALTONA RURAL WATER SERVICES CO-OP LTD ALTONA R0G $2,145.25 ARBORG BI-FROST PARKS & RECREATION COMMISSION ARBORG R0C $4,415.00 ARGYLE-LORNE-SOMERSET WEED CONTROL DISTRICT BALDUR R0K $322.95 ARLINGTONHAUS INC. WINNIPEG R2K $6,353.08 ARTEMIS HOUSING CO-OP LTD WINNIPEG R3A $1,573.30 ASTRA NON-PROFIT HOUSING CORPORATION WINNIPEG R2K $2,548.64 AUTUMN HOUSE INC. WINNIPEG R3E $7,366.31 BAPTIST MISSION APARTMENTS INC. WINNIPEG R3E $3,828.08 BARROWS COMMUNITY COUNCIL BARROWS R0L $2,713.78 BEAUSEJOUR BROKENHEAD DEVELOPMENT CORP BEAUSEJOUR R0E $4,669.11 BETELSTADUR HOUSING COOPERATIVE LTD WINNIPEG R3A $669.10 BETHANIAHAUS INC. WINNIPEG R2K $6,160.12 BIBLIOTHÉQUE MONTCALM LIBRARY SAINT-JEAN-BAPTISTE R0G $177.01 BIBLIOTHÉQUE REGIONALE JOLYS REGIONAL LIBRARY SAINT-PIERRE-JOLYS R0A $761.21 BIBLIOTHÉQUE TACHÉ LIBRARY LORETTE R0A $729.46 BISSETT COMMUNITY COUNCIL BISSETT R0E $4,346.40 BLUMENFELD HOCHFELD WATER CO-OP LTD WINKLER R6W $540.72 BLUMENORT SENIOR CITIZENS HOUSING INC. STEINBACH R5G $834.10 BOISSEVAIN -

Arctic Gateway Media Backgrounder
THE ARCTIC GATEWAY GROUP IS OWNED BY FIRST NATIONS AND BAYLINE COMMUNITIES, BUILDING A NATURAL RESOURCES CORRIDOR THROUGH THE ARCTIC TO THE WORLD. ABOUT THE ARCTIC GATEWAY GROUP The Arctic Gateway Group LP owns and operates the Port of Churchill, Canada’s only Arctic seaport serviced by rail, on the Hudson Bay Railway, running from The Pas to Churchill, Manitoba. Strategically located on the west coast of Hudson Bay, the Arctic Gateway is the front door to Western Canada, linking Canadian trade in resources to the global marketplace. HUDSON BAY RAILWAY The Arctic Gateway’s logistical advantage, rail assets and unique location provide direct and efficient routes to markets for Canada’s abundant natural resources and manufactured products, while connecting Canadian consumers and importers to the world marketplace via the North. THE ARCTIC GATEWAY OFFERS LOCAL COMMUNITIES... Local business contracting opportunities Local training and development initiatives to Lasting economic activity create lasting wealth and prosperity for Northern Employment Manitoba First Nations and communities Arctic Gateway Group LP Arctic_Gateway ArcticGateway 728 Bignell Ave. ArcticGateway The Pas, MB R9A 1L8 1-888-445-1112 [email protected] www.arcticgateway.com PORT OF CHURCHILL trategically located on the west coast Open Water Days Per Year S of Hudson Bay, the Port of Churchill 200 Average Increase: 1.14 days/year brings Atlantic Ocean trade to the doorstep 180 of Western Canada. 160 The Port of Churchill offersfour deep-sea berths for loading 140 and unloading grain, manufactured, mining and forest 120 commodities, general cargo, and tanker vessels. Connected Season (Days) of Open Water Length to the rest of Canada via the Hudson Bay Railway, the Port 1985 1990 1995 2000 2005 2010 of Churchill is closer to 25% of Canada’s western grain production than any other port in the world.