CHIM0698 Surface Tension
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
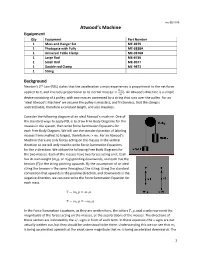
Atwood's Machine? (5 Points)
rev 09/2019 Atwood’s Machine Equipment Qty Equipment Part Number 1 Mass and Hanger Set ME‐8979 1 Photogate with Pully ME‐6838A 1 Universal Table Clamp ME‐9376B 1 Large Rod ME‐8736 1 Small Rod ME‐8977 1 Double rod Clamp ME‐9873 1 String Background Newton’s 2nd Law (NSL) states that the acceleration a mass experiences is proportional to the net force applied to it, and inversely proportional to its inertial mass ( ). An Atwood’s Machine is a simple device consisting of a pulley, with two masses connected by a string that runs over the pulley. For an ‘ideal Atwood’s Machine’ we assume the pulley is massless, and frictionless, that the string is unstretchable, therefore a constant length, and also massless. Consider the following diagram of an ideal Atwood’s machine. One of the standard ways to apply NSL is to draw Free Body Diagrams for the masses in the system, then write Force Summation Equations for each Free Body Diagram. We will use the standard practice of labeling masses from smallest to largest, therefore m2 > m1. For an Atwood’s Machine there are only forces acting on the masses in the vertical direction so we will only need to write Force Summation Equations for the y‐direction. We obtain the following Free Body Diagrams for the two masses. Each of the masses have two forces acting on it. Each has its own weight (m1g, or m2g) pointing downwards, and each has the tension (T) in the string pointing upwards. By the assumption of an ideal string the tension is the same throughout the string. -

Physics 114 Tutorial 5: Tension Instructor: Adnan Khan
• Please do not sit alone. Sit next to at least one student. • Please leave rows C, F, J, and M open. Physics 114 Tutorial 5: Tension Instructor: Adnan Khan 5/7/19 1 Blocks connected by a rope q Section 1: Two blocks, A and B, are tied together with a rope of mass M. Block B is being pushed with a constant horizontal force as shown at right. Assume that there is no friction between the blocks and the blocks are moving to the right. 1. Describe the motion of block A, block B, and the rope. 2. Compare the acceleration of block A, block B and the rope. 5/7/19 2 Blocks connected by a rope 2. Compare the accelerations of block A, block B and the rope. A. aA > aR > aB B. aB > aA > aR C. aA > aB > aR D. aB > aR > aA E. aA = aR = aB 5/7/19 3 Blocks connected by a rope 3. Draw a separate free-body diagram for each block and for the rope. Clearly label your forces. 5/7/19 4 Blocks connected by a rope 4. Rank, from largest to smallest, the magnitudes of the horizontal components of the forces on your diagrams. 5/7/19 5 Blocks connected by a very light string q Section 2: The blocks in section 1 are now connected with a very light, flexible, and inextensible string of mass m (m < M). Suppose the hand pushes so the acceleration of the blocks is the same as in section 1. Blocks have same acceleration as with rope 5. -

Vibration Suppression in Simple Tension-Aligned Structures
VIBRATION SUPPRESSION IN SIMPLE TENSION-ALIGNED STRUCTURES By Tingli Cai A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree of Mechanical Engineering – Doctor of Philosophy 2016 ABSTRACT VIBRATION SUPPRESSION IN SIMPLE TENSION-ALIGNED STRUCTURES By Tingli Cai Tension-aligned structures have been proposed for space-based antenna applications that require high degree of accuracy. This type of structures use compression members to impart tension on the antenna, thus helping to maintain the shape and facilitate disturbance rejection. These struc- tures can be very large and therefore sensitive to low-frequency excitations. In this study, two control strategies are proposed for the purpose of vibration suppression. First, a semi-active con- trol strategy for tension-aligned structures is proposed, based on the concept of stiffness variation by sequential application and removal of constraints. The process funnels vibration energy from low-frequency to high-frequency modes of the structure, where it is dissipated naturally due to internal damping. In this strategy, two methods of stiffness variation were investigated, including: 1) variable stiffness hinges in the panels and 2) variable stiffness elastic bars connecting the panels to the support structure. Two-dimensional and three-dimensional models were built to demon- strate the effectiveness of the control strategy. The second control strategy proposed is an active scheme which uses sensor feedback to do negative work on the system and to suppress vibration. In particular, it employs a sliding mechanism where the constraint force is measured in real time and this information is used as feedback to prescribe the motion of the slider in such a way that the vibration energy is reduced from the structure continuously and directly. -

Glossary of Materials Engineering Terminology
Glossary of Materials Engineering Terminology Adapted from: Callister, W. D.; Rethwisch, D. G. Materials Science and Engineering: An Introduction, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, 2010. McCrum, N. G.; Buckley, C. P.; Bucknall, C. B. Principles of Polymer Engineering, 2nd ed.; Oxford University Press: New York, NY, 1997. Brittle fracture: fracture that occurs by rapid crack formation and propagation through the material, without any appreciable deformation prior to failure. Crazing: a common response of plastics to an applied load, typically involving the formation of an opaque banded region within transparent plastic; at the microscale, the craze region is a collection of nanoscale, stress-induced voids and load-bearing fibrils within the material’s structure; craze regions commonly occur at or near a propagating crack in the material. Ductile fracture: a mode of material failure that is accompanied by extensive permanent deformation of the material. Ductility: a measure of a material’s ability to undergo appreciable permanent deformation before fracture; ductile materials (including many metals and plastics) typically display a greater amount of strain or total elongation before fracture compared to non-ductile materials (such as most ceramics). Elastic modulus: a measure of a material’s stiffness; quantified as a ratio of stress to strain prior to the yield point and reported in units of Pascals (Pa); for a material deformed in tension, this is referred to as a Young’s modulus. Engineering strain: the change in gauge length of a specimen in the direction of the applied load divided by its original gauge length; strain is typically unit-less and frequently reported as a percentage. -

Pulleys Mc-Web-Mech2-12-2009 Various Modelling Assumptions Are Used When Solving Problems Involving Pulleys
Mechanics 2.12. Pulleys mc-web-mech2-12-2009 Various modelling assumptions are used when solving problems involving pulleys. The connecting string is generally taken to be light and inextensible. The pulley is assumed to be smooth and light. Worked Example 1. A light inextensible string passes over a smooth light pulley. At each end of the string there is a particle. Particle B has a mass of 8 kg and particle C has a mass of 2 kg as shown in the diagram. The particles are released from rest with the string taut. Calculate the tension in the string and the acceleration of the masses. Solution It is important to understand that the magnitude of the tension will be the same throughout the string. This is crucial in problems with connected particles. In addition, the acceleration of each particle is of the same magnitude, but in opposite directions. It is usual to consider each of the particles separately and drawing a separate force diagram for each particle can be beneficial. Using Newton’s second law, F = ma, on par- ticle B, in the direction of motion (↓), gives: 8g − T =8a (1) TT Using Newton’s second law, F = ma, on par- a BC a ticle C, in the direction of motion (↑), gives: 8g T − 2g =2a (2) 2g The simultaneous equations (1) and (2) can now be solved. From (2): T = 2a + 2g (3) Substituting (3) into (1) gives: 8g − 2a − 2g =8a 6 6g = 10a ⇒ a = g =5.9 m s−2 (2 s.f.) 10 It is then a simple matter to substitute this value of a back into one of the equations, say (3), to obtain 6 32 T =2 × g +2g = g = 31 N (2 s.f.) 10 10 Worked Example 2. -

Students' Difficulties with Tension in Massless Strings. Part I
ENSENANZA˜ REVISTA MEXICANA DE FISICA´ E 55 (1) 21–33 JUNIO 2009 Students’ difficulties with tension in massless strings. Part I. S. Flores-Garc´ıa, L.L. Alfaro-Avena, J.E. Chavez-Pierce,´ J. Estrada, and J.V. Barron-L´ opez´ Universidad Autonoma´ de Ciudad Juarez,´ Avenida del Charro 450 Nte. Col. Partido Romero 32310 Ciudad Juarez´ Chih. M.D. Gonzalez-Quezada´ Instituto Tecnologico´ de Ciudad Juarez,´ Avenida Tecnologico´ 1340, Fracc. Crucero 32500 Ciudad Juarez´ Chih. Recibido el 27 de marzo de 2008; aceptado el 27 de enero de 2009 Many students enrolled in the introductory mechanics courses have learning difficulties related to the concept of force in the context of tension in massless strings. One of the potential causes could be a lack of functional understanding through a traditional instruction. In this article, we show a collection of this kind of students’ difficulties at the New Mexico State University, at the Arizona State University, and at the Independent university of Ciudad Juarez in Mexico. These difficulties were collected during an investigation conducted not only in lab sessions but also in lecture sessions. The first part of the investigation is developed in the contexts of proximity and the length of strings. Keywords: Tension; forces in strings; learning difficulties; force as a tension. Muchos estudiantes de los cursos introductorios de mecanica´ presentan serias dificultades para comprender el concepto de fuerza como vector en el contexto de la tension´ en cuerdas de masa despreciable. Una de las posibles causas es la falta de entendimiento funcional desarrollado durante las clases fundamentadas en una ensenanza˜ tradicional. -

Lectures Notes on Mechanics of Solids
Lectures notes On Mechanics of Solids Course Code- BME-203 Prepared by Prof. P. R. Dash SYLLABUS Module – I 1. Definition of stress, stress tensor, normal and shear stresses in axially loaded members. Stress & Strain :- Stress-strain relationship, Hooke’s law, Poisson’s ratio, shear stress, shear strain, modulus of rigidity. Relationship between material properties of isotropic materials. Stress-strain diagram for uniaxial loading of ductile and brittle materials. Introduction to mechanical properties of metals-hardness,impact. Composite Bars In Tension & Compression:-Temperature stresses in composite rods – statically indeterminate problem. (10) Module – II 2. Two Dimensional State of Stress and Strain: Principal stresses, principal strains and principal axes, calculation of principal stresses from principal strains.Stresses in thin cylinder and thin spherical shells under internal pressure , wire winding of thin cylinders. 3. Torsion of solid circular shafts, twisting moment, strength of solid and hollow circular shafts and strength of shafts in combined bending and twisting. (10) Module – III 4. Shear Force And Bending Moment Diagram: For simple beams, support reactions for statically determinant beams, relationship between bending moment and shear force, shear force and bending moment diagrams. 5. Pure bending theory of initially straight beams, distribution of normal and shear stress, beams of two materials. 6. Deflection of beams by integration method and area moment method. (12) Module – IV 7. Closed coiled helical springs. 8. Buckling of columns : Euler’s theory of initially straight columns with various end conditions, Eccentric loading of columns. Columns with initial curvature. (8) 1 Text Books-: 1. Strength of materials by G. H. Ryder, Mc Millan India Ltd., 2. -

Lecture 8: Surface Tension, Internal Pressure and Energy of a Spherical Particle Or Droplet
Lecture 8: Surface tension, internal pressure and energy of a spherical particle or droplet Today’s topics • Understand what is surface tension or surface energy, and how this balances with the internal pressure of a droplet to determine the droplet size. • Why smaller droplets are not stable and tend to fuse into larger ones? So far, we have considered only bulk quantities of any given substance when describing its thermodynamic properties. The role of surfaces/interfaces is ignored. However, when the substance shrink in size down to a small droplet, particle, or precipitate, the effects of surface/interface is no longer negligible, since the number atoms on Surface/Interface is comparable to number of atoms inside bulk. Surface effects (surface energy) play critical role many kinetics process of materials, such as the particle ripening and nucleation, as we will learn the lectures later. Typically, for many liquids and solid, surface energy (surface tension) is in the order of ~ 1 J/m2 (or N/m). For example, for water under ambient conditions, the surface energy is 0.072 J/m2 (or surface tension of 0.072 N/m). Think about a large oil droplet suspending in a cup of water: shake the cup, the oil droplet will disperse as many small ones, but these small droplets will eventually fuse together, forming back to a large one if let the cup stabilize for a while. This is because the increased surface energy due to the smaller droplets (particles), fusing back to one large particle decreases the total energy, favorable in thermodynamic. Essentially every phase transformation begins with the formation of a tiny spherical particle called nucleus. -

Aeolian Vibration Basics EN-ML-1007-4
Aeolian Vibration Basics October 2013 Part of a series of reference reports prepared by Preformed Line Products Aeolian Vibration Basics Contents Page Abstract .............................................................................................................................1 Mechanism of Aeolian Vibrations ......................................................................................1 Effects of Aeolian Vibration ...............................................................................................3 Safe Design Tension with Respect to Aeolian Vibration....................................................5 Influence of Suspension Hardware....................................................................................5 Dampers: How they Work .................................................................................................6 Damper Industry Specifications.........................................................................................7 Damper: Laboratory Testing..............................................................................................8 Dampers: Field Testing .....................................................................................................9 ABSTRACT by the following relationship that is based on the Strouhal Number [2]. Wind induced (aeolian) vibrations of conductors and overhead shield wires Vortex Frequency (Hertz) = 3.26V / d (OHSW) on transmission and distribution lines can produce damage that will negatively where: V is the wind velocity component -

Small Phospholipid Vesicles: Internal Pressure, Surface Tension, and Surface Free Energy (Phospholipid Bilayer) STEPHEN H
Proc. Nati. Acad. Sci. USA Vol: 77, No. 7, pp. 4048-4050, July 1980 Biophysics Small phospholipid vesicles: Internal pressure, surface tension, and surface free energy (phospholipid bilayer) STEPHEN H. WHITE Department of Physiology and Biophysics, University of California, Irvine, California 92717 Communicated by John A. Clements, April 25,1980 ABSTRACT Tanford [Tanford, C. (1979) Proc. NatL Acad. SURFACE TENSION AND LAPLACE'S LAW Sci. USA 76, 3318-33191 used thermodynamic arguments to show that the pressure difference across the bilayer of small Despite the fact that surfaces consist of thin layers a few mol- phospholipid vesicles must be zero. This paper analyzes the ecules thick, surface tension is a macroscopic quantity because implications of this conclusion in terms of Laplace's law and it is determined by some macroscopic means, such as a mea- the basic thermodynamics of interfaces. In its usual form, La- surement of the force exerted by a surface phase on a wire place's law is of questionable value for the vesicle. If the vesicle frame or a platinum plate. Surface tension can be defined is in a state of metastable equilibrium, the surface free energy must be minimal with respect to several thermodynamic vari- thermodynamically as (OF/OA)TV, but a more useful definition ables; the condition (OF/OA) = 0 is not adequate by itself. for my purposes is that of Bakker (3, 4), given by Tanford (1) has raised a question of fundamental importance e = f P2[P(Z) - PIZ)JdZ. [31 to understanding the physical chemistry of small phospholipid vesicles of the type first described by Huang (2). -

Section 13 – Tension in Ropes with Pulleys
Physics 204A Class Notes Section 13 – Tension in Ropes with Pulleys Outline 1. Examples with Ropes and Pulleys We are still building our understanding of why do objects do what they do – in terms of forces. In this section we will look at the behavior of systems that involve ropes and pulleys. Note that many of these examples involve multiple systems. 1. Examples with Ropes and Pulleys For a light string or rope, we have assumed that the tension is transmitted undiminished throughout the rope. We will continue to assume this to be true even when the rope changes direction due to a light frictionless pulley. When a pulley changes the direction of motion some complications need to be addressed. Example 13.1: A 0.500kg mass is connected by a string to a second mass as shown at the right. The 500g mass accelerates downward at 1.20m/s2. Find (a)the other mass and (b)the tension in the string. Given: M = 0.500kg and a = 1.20m/s2. Find: m = ? and Ft = ? This device is called an “Atwood Machine.” The easiest way to deal with pulleys is to choose (and stick with) a consistent coordinate system. In this problem, let’s chose clockwise motion as positive. Now, we’ll look separately at the forces on each mass. Applying the Second Law to the 500g mass, Ft Ft ΣF = ma ⇒ Fg − Ft = Ma ⇒ Mg − Ft = Ma ⇒ Ft = Mg − Ma . Note that the force of gravity is positive and the tension is negative using the chosen coordinates. Plugging in to find the tension, Ft = (0.5)(9.8) − (0.5)(1.2) ⇒ Ft = 4.3N . -

Circular Motion in “Vertical” Circles
Circular Motion, Day 5 Circular Motion in “Vertical” Circles So, let’s re-cap a few quick points. When a ball is swung on a string in a vertical circle, the tension is greatest at the bottom of the circular path. This is where the rope is most likely to break. It should make sense that the tension at the bottom is the greatest. Imagine watching the summer Olympics, specifically the gymnastics competitions. When a gymnast is doing the parallel bars, spinning themselves around in a vertical circle, when does she have to struggle the most to keep hold of the bar? Not at the top. In fact, at the top the gymnast feels relief (and often repositions her hands). At the bottom, however, the gymnast’s arms will hurt. Gymnasts are most likely to lose hold of the parallel bar when reaching the bottom of their circular path. However, what happens if the ball on the string (or the gymnast, for that matter) isn’t going very fast. Without enough speed, the ball won’t be able to complete the circle. It will leave its circular path at the top, because the string will lose tension. This can be seen in the diagram at the right. So, a MINIMUM VELOCITY is needed for objects to complete vertical circles. How do you find this minimum velocity? Well……if the ball “just barely” gets over the top of the circle, the tension in this “just barely” case will be “just barely” greater than zero at the top. After all, the key to swinging something in a vertical circle is to keep tension in the rope.