Vattikuti Institute Prostatectomy (Vip) and Current Results
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mega Prize Winners VIKAS MAHAJAN SAMIR SHELAR UBAID AHMED K DILIP JAIN N KAVYA ASHISH AHIR RAJVARDHAN S LODHA FAIZAL KOTTIKOLLON
Mega Prize Winners VIKAS MAHAJAN SAMIR SHELAR UBAID AHMED K DILIP JAIN N KAVYA ASHISH AHIR RAJVARDHAN S LODHA FAIZAL KOTTIKOLLON SUMIT JAIN DHARMEN JADIA SAHIL TUTEJA SEJAL MODY Weekly Winners POONAMCHAND JAIN PRADEEP SHARMA SATISH WAGLE CYRUS P MISTRY JAGDISH PRASAD BANSAL SANDEEP JAIN SUSHIL KUMAR JAIN AAKRITI JAIN AYAAN SHETTY MITHLESH CHAUHAN SUNIL T KUKREJA SAMBHAJI KOLTE CHANDAN BHOWMICK SANJAY KAPUR NIKHIL MITTAL MOHIT RATHOD APURVA SHAH AMIT KOTHARI R BALAJI RANJITH S R DR GAURAV BASUTKAR SHARAD AGGARWAL CHETAN PRAJAPATI ANANT MEHTA MANIK AGGARWAL SUNIL NIKOSE MADAN DESHPANDE SUNIL SIPANI NITIN GUPTA AMIT RAMAN ARORA RAVI BHOSHAN SINGH AMIT HINDUJA ROHAN KOTHARI ATUL MARDIA BHOPAL RAJPUT ADITYA KUMAR RAI ACHAL KRISHNA SIDDHARTH MEHTA NITIN SINGHAL MUKUND PATEL SUBHAJYOT MUKHERJEE RAJ RANI RAJEEV SAMANT SEEMA ANIKET KUMAR BHARAT TAK SHASHI CHOUDHARY ALOK KHANNA RAJENDER SAHIL SHARMA PRASAD VOGOTI TRACEY LOBO ANUJ MEHTA G KHWAJA MUNSHI RUPAK HALDER SAUD MIRZA GOPINATH SUDARSHAN KUMAR V S SRINIVAS S G GOVIND ABHIJEET SINGH RAJEEV MARATHE SHUVAYU BISWAS VENUGOPAL SUNKU Mahendra Kumar Rao MONIKA KHUNGAR BIJESHWAREE SHAH SIREESHA GIRISH MANOHAR LAL KUMAWAT SANGITA SHARMA ROHIT GUPTA ANITESH GIRI GOSWAMI RAMAMOHAN W V ANJALI GUPTA KARAN KUMAR BHUTOR NITABEN PATEL KRISHNAKANT GUPTA HAFEEZHUSSAIN SYED IZATPAL SINGH YUSUF MOHAMMED JAVED SUMIT KAPOOR SOURAV SINGLA VIJAYA SAGAR REDDY RAHUL KAPOOR VARGHESE ISAAC AVINASH MISHRA R NAGESWARA RAO S DORAIRAJ NAVDEEP CHAWLA SUSHIL KUMAR JAIN SNEHA RANJAN CHOUDHUR SHARAD BANSAL KAMLESH MAHESWARI KAILASH -

Past, Present and Future of Urological Robotic Surgery
Review Article ICUrology 2016;57:75-83. http://dx.doi.org/10.4111/icu.2016.57.2.75 pISSN 2466-0493 • eISSN 2466-054X Past, present and future of urological robotic surgery Wooju Jeong, Ramesh Kumar, Mani Menon Vattikuti Urology Institute, Henry Ford Health System, Detroit, MI, USA The first urologic robotic program in the world was built at the Vattikuti Urology Institute, Henry Ford Hospital Detroit, Michigan, in 2000 under the vision of surgical innovator, Dr. Mani Menon for the radical prostatectomy. The robot-assisted radical prostatec- tomy continues being modified with techniques to improve perioperative and surgical outcomes. The application of robotic surgi- cal technique has since been expanded to the bladder and upper urinary tract surgery. The evolution of surgical technique and its expansion of application will continue to improve quality, outcome parameters and experience for the patients. Keywords: Cystectomy; Nephrectomy; Prostatectomy; Robotics This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. A PERFECT STORM informed his foresight in seeking to apply the surgical robot to urologic surgery. With the generous support from the Raj The Vattikuti Urology Institute (VUI) at Henry Ford and Padma Vattikuti Foundation, a perfect storm gathered Hospital in Detroit, Michigan, USA established the first to initiate our journey into the robotic era. urologic robotic surgery program in the world in 2000. It would not have happened without 3 crucial factors: an RADICAL PROSTATECTOMY innovative idea, an experienced surgeon with tremendous foresight, and considerable philanthropic funding. -
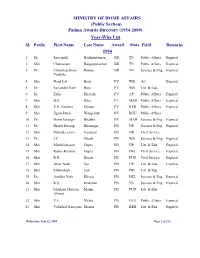
(Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl
MINISTRY OF HOME AFFAIRS (Public Section) Padma Awards Directory (1954-2009) Year-Wise List Sl. Prefix First Name Last Name Award State Field Remarks 1954 1 Dr. Sarvapalli Radhakrishnan BR TN Public Affairs Expired 2 Shri Chakravarti Rajagopalachari BR TN Public Affairs Expired 3 Dr. Chandrasekhara Raman BR TN Science & Eng. Expired Venkata 4 Shri Nand Lal Bose PV WB Art Expired 5 Dr. Satyendra Nath Bose PV WB Litt. & Edu. 6 Dr. Zakir Hussain PV AP Public Affairs Expired 7 Shri B.G. Kher PV MAH Public Affairs Expired 8 Shri V.K. Krishna Menon PV KER Public Affairs Expired 9 Shri Jigme Dorji Wangchuk PV BHU Public Affairs 10 Dr. Homi Jehangir Bhabha PB MAH Science & Eng. Expired 11 Dr. Shanti Swarup Bhatnagar PB UP Science & Eng. Expired 12 Shri Mahadeva Iyer Ganapati PB OR Civil Service 13 Dr. J.C. Ghosh PB WB Science & Eng. Expired 14 Shri Maithilisharan Gupta PB UP Litt. & Edu. Expired 15 Shri Radha Krishan Gupta PB DEL Civil Service Expired 16 Shri R.R. Handa PB PUN Civil Service Expired 17 Shri Amar Nath Jha PB UP Litt. & Edu. Expired 18 Shri Malihabadi Josh PB DEL Litt. & Edu. 19 Dr. Ajudhia Nath Khosla PB DEL Science & Eng. Expired 20 Shri K.S. Krishnan PB TN Science & Eng. Expired 21 Shri Moulana Hussain Madni PB PUN Litt. & Edu. Ahmed 22 Shri V.L. Mehta PB GUJ Public Affairs Expired 23 Shri Vallathol Narayana Menon PB KER Litt. & Edu. Expired Wednesday, July 22, 2009 Page 1 of 133 Sl. Prefix First Name Last Name Award State Field Remarks 24 Dr. -

Academy News
Proc Indian Natn Sci Acad 85 No. 4 December 2019 pp. 1067-1090 Printed in India. ACADEMY NEWS INSA MEETINGS to Professor Tarun Kant, FNA, Professor Emeritus, Department of Civil Engineering, Several meetings were held during April 08-10, 2019 Indian Institute of Technology Bombay, Powai, in the Academy premises. Mumbai. These included meetings of the different Sectional 4. Professor K Naha Memorial Medal to Committees for recommending names of Young Professor SS Rai, FNA, Professor Emeritus, Scientist Awardees and for the first round of short- Department of Earth and Climate Science, listing of nominations for INSA Fellowship. The Indian Institute of Science Education & Advisory Boards for the various INSA Awards also Research (IISER), Pune. met. These were followed by meetings of the Council and General Body. (C) Endowment Lectures INSA Medal/Lecture Awards 2019 5. Professor Darshan Ranganathan Memorial Lecture to Professor Gaiti Hasan, FNA, The Academy at its General Body Meeting on April National Centre for Biological Sciences, Tata 10, 2019 announced the following six medal/lecture Institute of Fundamental Research, Bengaluru. Awards for 2019. In addition, a new endowment award named International Award Professor TV Desikachary Memorial Medal was also 1. PMS Blackett Memorial Lecture to Sir Tom instituted. The Medal will be awarded to an eminent L Blundell, FNA, Emeritus Professor and scientist for his outstanding contributions in any area Director of Research, Department of of Biological Sciences. The award carries an Biochemistry, University of Cambridge, honorarium of Rs. 25,000/, a bronze medal and a Cambridge. citation. The first medal will be awarded in 2020. -

Program Book
SEPTEMBER 7–92006 northeastern Section of the AUA Scientific PROGRA M 2 Astellas Product Ad Cover 2 TABLE OF CONTENTS Schedule at a Glance . 1-3 Officers and Committee Lists . 4 Supporter Recognition. 5 Research and Education Fund . 6-7 George E. Slotkin Lecturers. 8 Guest Speakers . 9–13 Publication of Abstracts . 14 Program . 15 –25 Nursing Program . 26 Social Event Info: Dine Around. 27–28 Social Event Info: Tours. 29–30 Social Event Info: Events . 31–33 Exhibitor Listing (alpha) . 34–38 Exhibitor Listing (by Booth Number) . 39 Disclosure Information . 40–41 CME Form. 42 REGISTER ON LINE AT WWW.AUANET.ORG/NORTHEASTERN/ CONTINUING MEDICAL EDUCATION Accreditation Educational Goals and Objectives The American Urological Association Education and Research, Inc. is The Annual Meeting of the Northeastern Section of the American accredited by the Accreditation Council for Continuing Medical Education Urological Association, Inc. is designed to provide a forum for communi- (ACCME) to provide continuing medical education for physicians. The cating to the members and guest physicians the developing state of the American Urological Association Education and Research, Inc. takes art of science of urological techniques, evaluations and procedures. The responsibility for the content, quality and scientific integrity of this CME program will include original papers presenting new information address- activity. ing pertinent clinical topics. Panel discussion and point-counterpoint ses- sions on topical issues allow for participants to openly discuss procedure CME Credits and techniques. Participants will have the opportunity to discuss presen- tations and address questions to authors. At the completion of the The American Urological Association Education and Research, Inc. -

Dormant Account 10 Years and Above As on Ashadh 2076
Everest Bank Limited Head Office, Lazimpat 14th Aug 2019 DORMANT ACCOUNT 10 YEARS AND ABOVE AS ON ASHADH 2076 SN A/C NAME CURRENCY 1 BIRENDRA & PUNAMAYA EUR 2 ROBERT PRAETZEL EUR 3 NABARAJ KOIRALA EUR 4 SHREE NAV KANTIPUR BAHUUD NPR 5 INTERCONTINENTAL KTM HOTE NPR 6 KANHIYALAL & RAJESH OSWAL NPR 7 LAXMI HARDWARE NPR 8 NAVA KSHITIZ ENTERPRISES NPR 9 SWADESHI VASTRA BIKRI BHA NPR 10 UNNAT INDUSTRIES LTD. NPR 11 RAJ PHOTO STUDIO NPR 12 RND ENTERPRISES NPR 13 LUMBINI RESORT AND HILL D NPR 14 ARUNODAY POLYPACK IND. NPR 15 UNNAT INDUSTRIES PVT.LTD. NPR 16 KRISHNA MODERN DAL UDYOG NPR 17 TIRUPATI DISTRIB. CONCERN NPR 18 LAXMI GALLA KATTA KHARID NPR 19 URGN HARDWARE CONCERN. NPR 20 AASHMA COOPERATIVE FINANC NPR 21 VERITY PRINTERS(P)LT NPR 22 PUZA TRADERS NPR 23 NEPAL MATCH MANUFACTURER NPR 24 G.B TEXTILE MILLS PVT. LT NPR 25 VISION 9PRODUCT. (P) L.-R NPR 26 PHOOLPATI ENTERPRISES NPR 27 ROSHI SAVING & CREDIT CO. NPR 28 SHRESTHA TRD.GROUP P.LTD. NPR 29 P.D.CONSULT (PARTNERS FOR NPR 30 N.Y.S.M.S RELIEF FUND NPR 31 ASHOK WIRE PVT. LTD NPR 32 PAWAN KRISHNA HARDWARE ST NPR 33 OCEAN COMPUTER PVT. LTD. NPR 34 CHHIGU MULTIPURPOSE CO-OP NPR 35 GAJENDRA TRADERS NPR 36 SURENDRA KARKI NPR 37 GATE WAY INT'L TRADERS NPR 38 VYAHUT COMMERCIAL TRADERS NPR 39 SAPTA KOSHI SAV.&CR. CO.L NPR 40 MHAIPI HOSIERY NPR 41 STYLE FOOTWEARS P LTD NPR 42 MINA IMPEX NPR 43 CUSTOM CLEARANCE SERVICE NPR 44 HOTEL LA DYNASY PVT. -

Generalizability of Prostate-Specific Antigen (PSA) Screening Trials in a "Real World" Setting: a Nationwide Survey Analysis
Henry Ford Health System Henry Ford Health System Scholarly Commons Urology Articles Urology 11-19-2020 Generalizability of Prostate-Specific Antigen (PSA) Screening Trials in a "Real World" Setting: A Nationwide Survey Analysis Deepansh Dalela Akshay Sood Jacob Keeley Craig G. Rogers Mani Menon See next page for additional authors Follow this and additional works at: https://scholarlycommons.henryford.com/urology_articles Authors Deepansh Dalela, Akshay Sood, Jacob Keeley, Craig G. Rogers, Mani Menon, and Firas Abdollah ARTICLE IN PRESS Commentary Generalizability of Prostate-Specific Antigen (PSA) Screening Trials in a “Real World” Setting: A Nationwide Survey Analysis Deepansh Dalela, Akshay Sood, Jacob Keeley, Craig Rogers, Mani Menon, and Firas Abdollah COMMENTARY Overall, 14,941 (weighted n = 15,405,057) men under- he Prostate, Lung, Colorectal and Ovarian cancer went annual PSA screening within the available years. (PLCO) and the European Randomized Study of The median (interquartile range) age for these men was Screening for Prostate Cancer (ERSPC) trials 1,2 61 (53.3-69.0) years, compared to 60.3 in ERSPC. Inter- T ≤ have been widely used to inform policy decisions regard- estingly, 43% of men were aged 60 in our cohort, vs < ing prostate-specific antigen (PSA) screening in the US. 32% in PLCO [P .001; PLCO only reported the percen- However, the generalizability of any trial to its intended tages in each age category; Fig. 1A]. Sensitivity analyses < population is directly contingent on the study subjects showed that the majority of men were aged 60 at their fi being "representative" of the "universe" (ie, US men with- rst PSA screening test (34% aged 40-49, 23.8% aged 50- out symptoms or known diagnosis of prostate cancer 54 and 13.5% aged 55-59). -

Jaiprakash Associates Limited
JAIPRAKASH ASSOCIATES LIMITED Detail of Dividend outstanding for consecutive seven years Dividend Dividend Amount Amount Folio No/ Holding as on Sl No 1st HolderName already outstanding DPID/CLIENTID 30.06.2017 transferred to with the IEPF Account Company 1 IN30021412632332 A BOOBALAN 108 4.70 17.78 2 IN30113513221854 A A GANAPATHY 487 305.50 1,139.58 3 IN30177410328616 A ANITHA 6 3.76 14.04 4 1201060001734581 A BENYAMIN BORAH 35 - 103.52 5 1244765 A BHASKER RAJ 7 4.70 16.38 6 1092427 A BHUVANESHWARI 7 4.70 16.38 7 1082022 A C DHUPAR 45 28.20 105.30 8 1154255 A C THOMAS 937 - 2,192.58 9 1099197 A CHITRA 1222 766.10 2,859.48 10 1043547 A F.GNANAMUTHU 7 4.70 16.38 11 1078421 A G SANTHANAM 420 263.20 982.80 12 1203600000360918 A G SUJAY 100 - 44.20 13 1054789 A G.AMBIKUTTAN 187 - 437.58 14 1009163 A GANAPATHI 1545 968.20 3,615.30 15 IN30039416466829 A GUNASEELAN 15 - 35.10 16 IN30177413814722 A IRUDAYARAJ 15 - 35.10 17 1187063 A J JAMES 562 352.50 1,315.08 18 1086757 A J PRASHANTH 15 9.40 35.10 19 IN30021411658423 A JAGANNATHAN 98 1.88 67.02 20 1204470005048261 A JOSEPHSELVARAJ 15 - 16.70 21 1204470001132419 A K BABU 29 13.20 114.66 22 IN30072410082723 A K GUPTA 15 9.40 35.10 23 1201910100910222 A K PAL 25 - 58.50 24 2328395 A K SINGH 150 94.00 351.00 25 IN30177411368140 A K UPADHAYA 562 - 1,315.08 26 IN30226911017070 A K VENKATESWARA RAO 6 3.76 14.04 27 IN30177415353140 A KANDASAMY 33 - 77.22 28 1210279 A KUMAR BHATNAGAR 900 564.00 2,106.00 29 1204470002986527 A LAKSHMIDEVI 2 - 12.36 30 1204470003513905 A MANIKANDAN 1 - 13.58 31 1204000000070444 A MANJULA . -
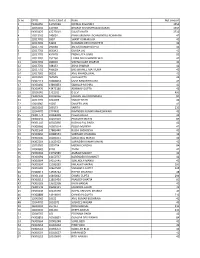
Sno Dpid Folio/Clid Name Net Amount 1 120765 66 G V SANDHYA 42.00
Sr no DP ID Folio /client id Name Net amount 1 IN300450 13797089 NIRMAL D GANDHI 1554 2 12033200 134939 BHARAT KISHANPRASAD DARAK 1554 3 IN300476 43273019 SULATA NATH 1712 4 12013200 245001 PANKAJKUMAR LACHMANDAS ACHHNANI 42 5 12017701 1897 SHANT KUMAR JAIN 42 6 12017701 53266 RUKMANI DEVI KHUNTETA 42 7 12017701 292048 ASHOK KUMAR GUPTA 42 8 12017701 666342 DURGA LAL 42 9 12017701 457678 PREM LATA GARG 82 10 12017701 252760 LAXMI DEVI KHANDELWAL 42 11 12017701 289630 VISHNU KANT SHARMA 42 12 12017701 348153 ASHA SHARMA 42 13 12017701 496629 BRIJ BIHAREE MATHURIA 42 14 13017600 90292 ANIL KHANDELWAL 42 15 12033500 969595 ALKA GUPTA 42 16 IN302113 10069824 USHA RANI RITHALIYA 42 17 IN300450 13893853 SANKALP MISHRA 42 18 IN300476 43475180 ABHINAV GUPTA 42 19 12036000 1712011 D LEVI 400 20 IN302236 10012036 MADAN LAL MOONDHRA 84 21 12017701 1024008 SANJAY RATHI 42 22 12018902 15037 SANJEEV JAIN 42 23 16010100 269173 SARITA 210 24 12044700 2774490 RAVINDER KUMAR MAHESHWARI 42 25 IN301774 10068428 Pawan Anand 42 26 IN300142 10257504 PREKSHIT MEHTA 84 27 IN301143 10013856 KUSHAL PAL SINGH 42 28 IN300966 10502014 POOJA MUNDRA 42 29 IN301549 17883440 RUCHI SACHDEVA 42 30 IN300966 10084553 SUBHASH CHANDRA 12 31 IN300206 10281051 SURAJ MAL MANOT 42 32 IN302269 11625452 SURINDER KUMAR MAKIN 42 33 12033500 1859754 MEENU CHADHA 84 34 12049600 8218 TANVI 42 35 IN300394 13752585 AMRAW MANOT 42 36 IN300394 14150737 NARENDER KR MANOT 42 37 IN300394 14150745 SURENDER MANOT 42 38 IN300394 15039283 KAILASH THAKRAL 100 39 IN300183 10467538 SANGEETA GUPTA 128 -

How to Meet Competing Goals During Robotic Radical Prostatectomy
Laparoscopic & Robotic CANCER CONTROL AND PRESERVATION OF NEUROVASCULAR TISSUE TEWARI ET AL. Laparoscopic and Robotic Urology Associate Editor Cancer control and the preservation of Ash Tewari neurovascular tissue: how to meet Editorial Board competing goals during robotic Ralph Clayman, USA Inderbir Gill, USA radical prostatectomy Roger Kirby, UK Mani Menon, USA Ashutosh Tewari, Sandhya Rao, Juan I. Martinez-Salamanca, Robert Leung, Rajan Ramanathan, Anil Mandhani, E. Darracott Vaughan, Mani Menon*, Wolfgang Horninger†, Jiangling Tu‡ and Georg Bartsch† The New York-Presbyterian Hospital, Departments of Urology and ‡Pathology, Weill Medical College of Cornell University, New York, NY, *Vattikuti Urology Institute, Henry Ford Health System, Detroit, MI, USA, and †The Institute of Urology, Innsbruck, Austria Accepted for publication 27 September 2007 RESULTS Study Type – Therapy (case series) Level of Evidence 4 The athermal technique addresses concerns about the use of thermal energy and OBJECTIVE bulldog clamps during nerve sparing, and emphasizes the importance of the trizonal To present early functional and oncological neural architecture. We analysed the data for the athermal trizonal nerve-sparing surgical outcomes of 215 consecutive technique of robotic radical prostatectomy patients from January 2005. The operative (RP), that addresses the concerns about duration was 120–240 min and the mean deviations from the principles of open RP and blood loss was 150 mL. In patients potent revisits the anatomical foundations of this before RP the potency rate at 1 year after surgery from the robotic perspective. bilateral nerve-sparing was 87%. The overall surgical margin rate was 6.5% and positive PATIENTS AND METHODS margin rates for organ-confined cancer were 4.7%. -
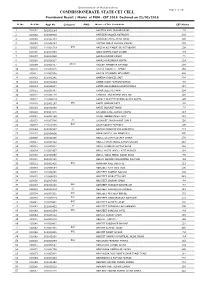
Provisional Result/Marks for PGM-CET 2016
Government of Maharashtra COMMISSIONERATE, STATE CET CELL Page 1 of 181 Provisional Result / Marks of PGM - CET 2016 Declared on 22/02/2016 Sr.No. Roll No. Appl No. Category PWD Name of The Candidate CET Marks 1 100001 161003184 AADITYA ANIL PRABHUDESAI 178 2 100002 161006430 AAFREEN NASSER KOTADIYA 201 3 100003 161011484 AAFREEN KAMAL AHAD AHAD 106 4 100004 161008664 AAFTAB ABRAR HUSAIN ANSARI 172 5 100005 161001529 OBC AAISHA ASHFAQUE GULREZ QADRI 109 6 100006 161009859 AAISHWARYA DILIP DHABE 159 7 100007 161003430 AAKASH RAJEN DOSHI 176 8 100008 161006337 AAKASH RAJENDRA GUPTA 124 9 100009 161001417 DT(VJ) AALEKH MANOHAR RATHOD 209 10 100010 161002045 AALIYA ASGAR ALI AFROZ 158 11 100011 161006308 AALIYA MEHMOOD MEHMOOD 208 12 100012 161001241 AAMERA SHAKEEL SAIT 228 13 100013 161002244 AAMIR JAKIR PATHAN PATHAN 165 14 100014 161006587 AAMIR ASHFAQUE BHARAPURWALA 117 15 100015 161000797 AAMIR JEELANI KHAN 204 16 100016 161000178 AANCHAL ANILKUMAR GVALANI 116 17 100017 161004219 AARSHI PRADEEP KUMAR GUPTA GUPTA 190 18 100018 161002167 OBC AARTI SOMVAR PATIL 188 19 100019 161001689 AARTI INDRAJIT MORE 176 20 100020 161007075 AARZOO SUNIL KUMAR VERMA 113 21 100021 161008750 AASHI ABOOBACKER AKAY 121 22 100022 161003786 SC AASHIKET SHASHIKANT SABLE 116 23 100023 161010785 OBC AASIM QASIM TAMBOLI 100 24 100024 161010517 AAYUSH PRADEEP KULSHRESTHA 213 25 100025 161004816 ABBAS HIBTULLAH MOAIYADI 205 26 100026 161010396 ABDUL GULAM MUSTAFA RAHUP 178 27 100027 161008422 ABDUL FAHIM ABDUL RAHIM SHAIKH 248 28 100028 161007061 ABDUL MANNAN SATTAR -

Robotic Radical Prostatectomy and the Vattikuti Urology Institute Technique: an Interim Analysis of Results and Technical Points
ROBOTIC RADICAL PROSTATECTOMY AND THE VATTIKUTI UROLOGY INSTITUTE TECHNIQUE: AN INTERIM ANALYSIS OF RESULTS AND TECHNICAL POINTS MANI MENON, ASHUTOSH TEWARI, AND MEMBERS OF THE VATTIKUTI INSTITUTE PROSTATECTOMY TEAM ABSTRACT We have performed Ͼ350 robotic radical prostatectomies in the last 2 years. A single surgeon (MM) performed 250 of these procedures using a technique developed at our institution, the Vattikuti Urology Institute. This article summarizes the technical highlights and interim results of the Vattikuti Institute Prostatectomy (VIP) technique. We prospectively collected baseline demographic data, such as age, race, body mass index (BMI), serum prostate-specific antigen values, prostate volume, Gleason score, percentage cancer, TNM clinical staging, and comorbidities. Urinary symptoms were measured with the International Prostate Symptom Score, and sexual health with the Sexual Health Inventory of Males. In addition, patients received the Expanded Prostate Inventory Composite at baseline and at 1, 3, 6, 12 and 18 months after the procedure via mail. Data collection is complete on 200 of the first 250 patient cases. Gleason score Ն7 was noted in 40% of patients. The average BMI was high (28), and 86% patients were classified as pathologic stage pT2a to pT2b. The mean operative time was 160 minutes and the mean blood loss was 153 mL. No patient required blood transfusion. At 6 months, 82% of the men who were Ͻ60 years of age and 75% of those Ͼ60 years of age had return of sexual function, and 64% and 38%, respectively, had sexual intercourse. At 6 months, 96% patients were continent. UROLOGY 61: 15–20, 2003. © 2003, Elsevier Science Inc.