Thesis Reference
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Variability of Major Phenyletanes and Phenylpropanoids in 16-Year-Old Rhodiola Rosea L
molecules Article Variability of Major Phenyletanes and Phenylpropanoids in 16-Year-Old Rhodiola rosea L. Clones in Norway Abdelhameed Elameen 1,* , Vera M. Kosman 2, Mette Thomsen 3, Olga N. Pozharitskaya 4 and Alexander N. Shikov 5 1 NIBIO, Norwegian Institute for Bioeconomy Research, Høghskoleveien 7, N-1431 Ås, Norway 2 St. Petersburg Institute of Pharmacy, Leningrad Region, Vsevolozhsky District, P 245 188663 Kuzmolovo, Russia; [email protected] 3 NIBIO, Norwegian Institute for Bioeconomy Research, Øst Apelsvoll, 2849 Kapp, Norway; [email protected] 4 Murmansk Marine Biological Institute of the Russian Academy of Sciences (MMBI RAS), Vladimirskaya, 17, 183010 Murmansk, Russia; [email protected] 5 St. Petersburg State Chemical Pharmaceutical University, Prof. Popov, 14, 197376 Saint-Petersburg, Russia; [email protected] * Correspondence: [email protected]; Tel.: +479-020-0875 Academic Editors: Francesco Cacciola and Lillian Barros Received: 17 June 2020; Accepted: 28 July 2020; Published: 30 July 2020 Abstract: Rhodiola rosea L. (roseroot) is an adaptogen plant belonging to the Crassulaceae family. The broad spectrum of biological activity of R. rosea is attributed to its major phenyletanes and phenylpropanoids: rosavin, salidroside, rosin, cinnamyl alcohol, and tyrosol. In this study, we compared the content of phenyletanes and phenylpropanoids in rhizomes of R. rosea from the Norwegian germplasm collection collected in 2004 and in 2017. In general, the content of these bioactive compounds in 2017 was significantly higher than that observed in 2004. The freeze-drying method increased the concentration of all phenyletanes and phenylpropanoids in rhizomes compared 1 with conventional drying at 70 ◦C. As far as we know, the content of salidroside (51.0 mg g− ) observed in this study is the highest ever detected in Rhodiola spp. -
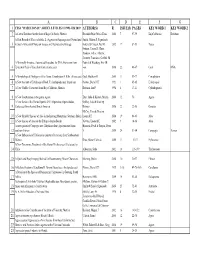
Haseltonia Articles and Authors.Xlsx
ABCDEFG 1 CSSA "HASELTONIA" ARTICLE TITLES #1 1993–#26 2019 AUTHOR(S) R ISSUE(S) PAGES KEY WORD 1 KEY WORD 2 2 A Cactus Database for the State of Baja California, Mexico Resendiz Ruiz, María Elena 2000 7 97-99 BajaCalifornia Database A First Record of Yucca aloifolia L. (Agavaceae/Asparagaceae) Naturalized Smith, Gideon F, Figueiredo, 3 in South Africa with Notes on its uses and Reproductive Biology Estrela & Crouch, Neil R 2012 17 87-93 Yucca Fotinos, Tonya D, Clase, Teodoro, Veloz, Alberto, Jimenez, Francisco, Griffith, M A Minimally Invasive, Automated Procedure for DNA Extraction from Patrick & Wettberg, Eric JB 4 Epidermal Peels of Succulent Cacti (Cactaceae) von 2016 22 46-47 Cacti DNA 5 A Morphological Phylogeny of the Genus Conophytum N.E.Br. (Aizoaceae) Opel, Matthew R 2005 11 53-77 Conophytum 6 A New Account of Echidnopsis Hook. F. (Asclepiadaceae: Stapeliae) Plowes, Darrel CH 1993 1 65-85 Echidnopsis 7 A New Cholla (Cactaceae) from Baja California, Mexico Rebman, Jon P 1998 6 17-21 Cylindropuntia 8 A New Combination in the genus Agave Etter, Julia & Kristen, Martin 2006 12 70 Agave A New Series of the Genus Opuntia Mill. (Opuntieae, Opuntioideae, Oakley, Luis & Kiesling, 9 Cactaceae) from Austral South America Roberto 2016 22 22-30 Opuntia McCoy, Tom & Newton, 10 A New Shrubby Species of Aloe in the Imatong Mountains, Southern Sudan Leonard E 2014 19 64-65 Aloe 11 A New Species of Aloe on the Ethiopia-Sudan Border Newton, Leonard E 2002 9 14-16 Aloe A new species of Ceropegia sect. -

LC-MS Profile, Gastrointestinal and Gut Microbiota
antioxidants Article LC-MS Profile, Gastrointestinal and Gut Microbiota Stability and Antioxidant Activity of Rhodiola rosea Herb Metabolites: A Comparative Study with Subterranean Organs Daniil N. Olennikov 1,* , Nadezhda K. Chirikova 2, Aina G. Vasilieva 2 and Innokentii A. Fedorov 3 1 Laboratory of Medical and Biological Research, Institute of General and Experimental Biology, Siberian Division, Russian Academy of Science, 6 Sakh’yanovoy Street, Ulan-Ude 670047, Russia 2 Department of Biology, Institute of Natural Sciences, North-Eastern Federal University, 58 Belinsky Street, Yakutsk 677027, Russia; [email protected] (N.K.C.); [email protected] (A.G.V.) 3 Institute for Biological Problems of Cryolithozone, Siberian Division, Russian Academy of Science, 41 Lenina Street, Yakutsk 677000, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-9021-600-627 Received: 26 May 2020; Accepted: 14 June 2020; Published: 16 June 2020 Abstract: Golden root (Rhodiola rosea L., Crassulaceae) is a famous medical plant with a one-sided history of scientific interest in the roots and rhizomes as sources of bioactive compounds, unlike the herb, which has not been studied extensively. To address this deficiency, we used high-performance liquid chromatography with diode array and electrospray triple quadrupole mass detection for comparative qualitative and quantitative analysis of the metabolic profiles of Rhodiola rosea organs before and after gastrointestinal digestion in simulated conditions together with various biochemical assays to determine antioxidant properties of the extracts and selected compounds. R. rosea organs showed 146 compounds, including galloyl O-glucosides, catechins, procyanidins, simple phenolics, phenethyl alcohol derivatives, (hydroxy)cinnamates, hydroxynitrile glucosides, monoterpene O-glucosides, and flavonol O-glycosides, most of them for the first time in the species. -

Opportunities and Pharmacotherapeutic Perspectives
biomolecules Review Anticoronavirus and Immunomodulatory Phenolic Compounds: Opportunities and Pharmacotherapeutic Perspectives Naiara Naiana Dejani 1 , Hatem A. Elshabrawy 2 , Carlos da Silva Maia Bezerra Filho 3,4 and Damião Pergentino de Sousa 3,4,* 1 Department of Physiology and Pathology, Federal University of Paraíba, João Pessoa 58051-900, Brazil; [email protected] 2 Department of Molecular and Cellular Biology, College of Osteopathic Medicine, Sam Houston State University, Conroe, TX 77304, USA; [email protected] 3 Department of Pharmaceutical Sciences, Federal University of Paraíba, João Pessoa 58051-900, Brazil; [email protected] 4 Postgraduate Program in Bioactive Natural and Synthetic Products, Federal University of Paraíba, João Pessoa 58051-900, Brazil * Correspondence: [email protected]; Tel.: +55-83-3216-7347 Abstract: In 2019, COVID-19 emerged as a severe respiratory disease that is caused by the novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). The disease has been associated with high mortality rate, especially in patients with comorbidities such as diabetes, cardiovascular and kidney diseases. This could be attributed to dysregulated immune responses and severe systemic inflammation in COVID-19 patients. The use of effective antiviral drugs against SARS-CoV-2 and modulation of the immune responses could be a potential therapeutic strategy for Citation: Dejani, N.N.; Elshabrawy, COVID-19. Studies have shown that natural phenolic compounds have several pharmacological H.A.; Bezerra Filho, C.d.S.M.; properties, including anticoronavirus and immunomodulatory activities. Therefore, this review de Sousa, D.P. Anticoronavirus and discusses the dual action of these natural products from the perspective of applicability at COVID-19. -

Effects of Dissolved Organic Matter from the Rhizosphere of the Hyperaccumulator
Journal of Hazardous Materials 192 (2011) 1616–1622 Contents lists available at ScienceDirect Journal of Hazardous Materials j ournal homepage: www.elsevier.com/locate/jhazmat Effects of dissolved organic matter from the rhizosphere of the hyperaccumulator Sedum alfredii on sorption of zinc and cadmium by different soils a,b,∗ a a b Tingqiang Li , Zhenzhen Di , Xiaoe Yang , Donald L. Sparks a Ministry of Education, Key Laboratory of Environmental Remediation and Ecosystem Health, College of Environmental and Resource Sciences, Zhejiang University, Hangzhou 310029, China b Department of Plant and Soil Sciences, University of Delaware, Newark, DE 19717, USA a r t i c l e i n f o a b s t r a c t Article history: Pot experiments were conducted to investigate the changes of the dissolved organic matter (DOM) in the Received 12 February 2011 rhizosphere of hyperaccumulating ecotype (HE) and non-hyperaccumulating ecotype (NHE) of Sedum Received in revised form 6 June 2011 alfredii and its effects on Zn and Cd sorption by soils. After planted with HE, soil pH in the rhizosphere Accepted 29 June 2011 reduced by 0.5–0.6 units which is consistent with the increase of DOM. The hydrophilic fractions (51%) Available online 5 July 2011 in DOM from the rhizosphere of HE (HE-DOM) was much greater than NHE-DOM (35%). In the presence of HE-DOM, Zn and Cd sorption capacity decreased markedly in the following order: calcareous clay Keywords: loam > neutral clay loam > acidic silty clay. The sorption isotherms could be well described by the Fre- Dissolved organic matter 2 Cadmium undlich equation (R > 0.95), and the partition coefficient (K) in the presence of HE-DOM was decreased by 30.7–68.8% for Zn and 20.3–59.2% for Cd, as compared to NHE-DOM. -

Qrno. 1 2 3 4 5 6 7 1 CP 2903 77 100 0 Cfcl3
QRNo. General description of Type of Tariff line code(s) affected, based on Detailed Product Description WTO Justification (e.g. National legal basis and entry into Administration, modification of previously the restriction restriction HS(2012) Article XX(g) of the GATT, etc.) force (i.e. Law, regulation or notified measures, and other comments (Symbol in and Grounds for Restriction, administrative decision) Annex 2 of e.g., Other International the Decision) Commitments (e.g. Montreal Protocol, CITES, etc) 12 3 4 5 6 7 1 Prohibition to CP 2903 77 100 0 CFCl3 (CFC-11) Trichlorofluoromethane Article XX(h) GATT Board of Eurasian Economic Import/export of these ozone destroying import/export ozone CP-X Commission substances from/to the customs territory of the destroying substances 2903 77 200 0 CF2Cl2 (CFC-12) Dichlorodifluoromethane Article 46 of the EAEU Treaty DECISION on August 16, 2012 N Eurasian Economic Union is permitted only in (excluding goods in dated 29 may 2014 and paragraphs 134 the following cases: transit) (all EAEU 2903 77 300 0 C2F3Cl3 (CFC-113) 1,1,2- 4 and 37 of the Protocol on non- On legal acts in the field of non- _to be used solely as a raw material for the countries) Trichlorotrifluoroethane tariff regulation measures against tariff regulation (as last amended at 2 production of other chemicals; third countries Annex No. 7 to the June 2016) EAEU of 29 May 2014 Annex 1 to the Decision N 134 dated 16 August 2012 Unit list of goods subject to prohibitions or restrictions on import or export by countries- members of the -

Sedum Society Newsletter(130) Pp
Open Research Online The Open University’s repository of research publications and other research outputs Kalanchoe arborescens - a Madagascan giant Journal Item How to cite: Walker, Colin (2019). Kalanchoe arborescens - a Madagascan giant. Sedum Society Newsletter(130) pp. 81–84. For guidance on citations see FAQs. c [not recorded] https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Version of Record Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk NUMBER 130 SEDUM SOCIETY NEWSLETTER JULY 2019 FRONT COVER Roy Mottram kindly supplied: “The Diet” copy of this Japanese herbal which is sharp and crisp (see page 97). “I counted the plates, and this copy is complete with 200 plates, in 8 parts, bound here in 2 vols. I checked for another Sedum but none are Established April 1987, now ending our present, so Maximowicz was basing his 32nd year. S. kagamontanum on this same plate, Subscriptions run from October to the following September. Anyone requesting translating the location as Mt. Kaga and to join after June, unless there is a special citing t.40 incorrectly. The "t.43" plate request, will receive his or her first number is also wrong. It is actually t.33 of Newsletter in October. If you do not the whole work, or Vol.2 t.8. The book is receive your copy by the 10th of April, July or October, or the 15th January, then bound back to front [by Western standards] please write to the editor: Ray as in all Japanese books of the day.” RM. -

Amorpha Fruticosa – a Noxious Invasive Alien Plant in Europe Or a Medicinal Plant Against Metabolic Disease?
fphar-08-00333 June 6, 2017 Time: 18:44 # 1 REVIEW published: 08 June 2017 doi: 10.3389/fphar.2017.00333 Amorpha fruticosa – A Noxious Invasive Alien Plant in Europe or a Medicinal Plant against Metabolic Disease? Ekaterina Kozuharova1, Adam Matkowski2, Dorota Wo´zniak2, Rumiana Simeonova3, Zheko Naychov4, Clemens Malainer5, Andrei Mocan6,7, Seyed M. Nabavi8 and Atanas G. Atanasov9,10,11* 1 Department of Pharmacognosy, Faculty of Pharmacy, Medical University of Sofia, Sofia, Bulgaria, 2 Department of Pharmaceutical Biology with Botanical Garden of Medicinal Plants, Medical University of Wroclaw, Poland, 3 Department of Pharmacology, Pharmacotherapy and Toxicology, Faculty of Pharmacy, Medical University of Sofia, Sofia, Bulgaria, 4 Sofia University St. Kliment Ohridski, Faculty of Medicine, Department of Surgery, Obstetrics and Gynecology, Division of Cardiac Surgery, University Hospital Lozenetz, Sofia, Bulgaria, 5 Independent Researcher, Vienna, Austria, 6 Department of Pharmaceutical Botany, Iuliu Ha¸tieganuUniversity of Medicine and Pharmacy, Cluj-Napoca, Romania, 7 ICHAT and Institute for Life Sciences, University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania, 8 Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran, 9 Institute of Genetics and Animal Breeding, Polish Academy of Sciences, Jastrzebiec, Poland, 10 Department of Pharmacognosy, University of Vienna, Vienna, Austria, 11 Department of Vascular Biology and Thrombosis Research, Center for Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria Amorpha fruticosa L. (Fabaceae) is a shrub native to North America which has been Edited by: Kalin Yanbo Zhang, cultivated mainly for its ornamental features, honey plant value and protective properties University of Hong Kong, Hong Kong against soil erosion. -

Flavonoid Glucodiversification with Engineered Sucrose-Active Enzymes Yannick Malbert
Flavonoid glucodiversification with engineered sucrose-active enzymes Yannick Malbert To cite this version: Yannick Malbert. Flavonoid glucodiversification with engineered sucrose-active enzymes. Biotechnol- ogy. INSA de Toulouse, 2014. English. NNT : 2014ISAT0038. tel-01219406 HAL Id: tel-01219406 https://tel.archives-ouvertes.fr/tel-01219406 Submitted on 22 Oct 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Last name: MALBERT First name: Yannick Title: Flavonoid glucodiversification with engineered sucrose-active enzymes Speciality: Ecological, Veterinary, Agronomic Sciences and Bioengineering, Field: Enzymatic and microbial engineering. Year: 2014 Number of pages: 257 Flavonoid glycosides are natural plant secondary metabolites exhibiting many physicochemical and biological properties. Glycosylation usually improves flavonoid solubility but access to flavonoid glycosides is limited by their low production levels in plants. In this thesis work, the focus was placed on the development of new glucodiversification routes of natural flavonoids by taking advantage of protein engineering. Two biochemically and structurally characterized recombinant transglucosylases, the amylosucrase from Neisseria polysaccharea and the α-(1→2) branching sucrase, a truncated form of the dextransucrase from L. Mesenteroides NRRL B-1299, were selected to attempt glucosylation of different flavonoids, synthesize new α-glucoside derivatives with original patterns of glucosylation and hopefully improved their water-solubility. -

Improved Cadmium Uptake and Accumulation in the Hyperaccumulator Sedum Alfredii: the Impact of Citric Acid and Tartaric Acid*
106 Lu et al. / J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2013 14(2):106-114 Journal of Zhejiang University-SCIENCE B (Biomedicine & Biotechnology) ISSN 1673-1581 (Print); ISSN 1862-1783 (Online) www.zju.edu.cn/jzus; www.springerlink.com E-mail: [email protected] Improved cadmium uptake and accumulation in the hyperaccumulator Sedum alfredii: the impact of *# citric acid and tartaric acid Ling-li LU†, Sheng-ke TIAN, Xiao-e YANG†‡, Hong-yun PENG, Ting-qiang LI (Ministry of Education Key Laboratory of Environmental Remediation and Ecological Health, College of Environmental and Resource Sciences, Zhejiang University, Hangzhou 310058, China) †E-mail: [email protected]; [email protected] Received Aug. 4, 2012; Revision accepted Jan. 17, 2013; Crosschecked Jan. 18, 2013 Abstract: The elucidation of a natural strategy for metal hyperaccumulation enables the rational design of tech- nologies for the clean-up of metal-contaminated soils. Organic acid has been suggested to be involved in toxic metallic element tolerance, translocation, and accumulation in plants. The impact of exogenous organic acids on cadmium (Cd) uptake and translocation in the zinc (Zn)/Cd co-hyperaccumulator Sedum alfredii was investigated in the present study. By the addition of organic acids, short-term (2 h) root uptake of 109Cd increased significantly, and higher 109Cd contents in roots and shoots were noted 24 h after uptake, when compared to controls. About 85% of the 109Cd taken up was distributed to the shoots in plants with citric acid (CA) treatments, as compared with 75% within controls. No such effect was observed for tartaric acid (TA). -

Morfološka Varijabilnost Vrste Sedum Album L. (Crassulaceae) Na Balkanskom Poluostrvu
UNIVERZITET U NIŠU PRIRODNO-MATEMATIĈKI FAKULTET DEPARTMAN ZA BIOLOGIJU I EKOLOGIJU MAJA D. JOVANOVIĆ Morfološka varijabilnost vrste Sedum album L. (Crassulaceae) na Balkanskom poluostrvu MASTER RAD Niš, 2017. UNIVERZITET U NIŠU PRIRODNO-MATEMATIĈKI FAKULTET DEPARTMAN ZA BIOLOGIJU I EKOLOGIJU Morfološka varijabilnost vrste Sedum album L. (Crassulaceae) na Balkanskom poluostrvu MASTER RAD Kandidat: Mentor: Maja D. Jovanović 206 Dr Bojan Zlatković Niš, 2017. UNIVERSITY OF NIŠ FACULTY OF SCIENCE AND MATHEMATICS DEPARTMENT OF BIOLOGY AND ECOLOGY Morphological variability of species Sedum album L. (Crassulaceae) on the Balkan Peninsula MASTER THESIS Candidate: Mentor: Maja D. Jovanović 206 Dr Bojan Zlatković Niš, 2017. BIOGRAFIJA KANDIDATA Maja D. Jovanović roĊena je u Nišu 27.03.1992. godine. Osnovnu školu ,,Stojan Ţivković Stole“ završava u Trnjanu (Aleksinac) nakon ĉega upisuje Srednju medicinsku školu ,,Milenko Hadţić“ (opšti smer) u Nišu. Prirodno-matematiĉki fakultet, Univerziteta u Nišu, upisuje 2011. godine, gde završava osnovne a potom i master studije, studijski program Ekologija i zaštita prirode, na Departmanu za biologiju i ekologiju. Tokom master studija uĉestvuje u promotivnim aktivnostima na projektima ,,Uspostavljanje konzervacionog menadţmenta slatina u Srbiji na osnovu monitoringa zajednice makroinvertebrata“ i ,,Uticaj lokalnog folklora i kulturnog nasleĊa na odnos ĉoveka prema šumskoj kornjaĉi (Testudo hermanii) u Srbiji“, finansiranih od strane Rufford Fondation kao i projektu ,,Divlji Viki Parkovi“ podrţanog od strane organizacije Vikimedija Srbije. Zahvalnica Neizmernu zahvalnost dugujem svom mentoru, Dr Bojanu Zlatkoviću, na ukazanom znanju, strpljenju, izvanrednoj saradnji i stručnom usmeravanju tokom godina studija i izrade ovog rada. Takođe, veoma sam zahvalna svim istraživačima koji su pomogli u sakupljanju biljnog materijala iz različitih delova Balkanskog poluostrva, i time doprineli da ovaj rad bude sveobuhvatniji. -

Final Project Report (To Be Submitted by 30Th September 2016)
Final Project Report (to be submitted by 30th September 2016) Instructions: Document length: maximum 10 pages, excluding this cover page and the last page on project tags. Start with an abstract (max 1 page). Final report text: Do not forget to mention your methodology; the people involved (who, how many, what organization they are from – if applicable); and the expected added value for biodiversity, society and the company. Finally, state whether the results of your project can be implemented at a later stage, and please mention the ideal timing and estimated costs of implementation. Annexes are allowed but will not be taken into account by the jury and must be sent separately. Word/PDF Final Report files must be less than 10 MB. If you choose to submit your final report in your local language, you are required to also upload your final report in English if you wish to take part in the international competition. To be validated, your file must be uploaded to the Quarry Life Award website before 30th September 2016 (midnight, Central European Time). To do so, please log in, click on ‘My account’/ ‘My Final report’. In case of questions, please liaise with your national coordinator. 1. Contestant profile . Contestant name: Moldovan Ștefan . Contestant occupation: Ornithologist . University / Organisation Romanian Ornithological Society . E-mail: . Phone (incl. country code): . Number of people in your team: 7 2. Project overview Title: Search for the critically endangered Apollo Butterfly at Bicaz Chei Quarry Contest: Quarry Life Award Quarry name: Bicaz Chei Quarry Prize category: ☐ Education and Raising Awareness (select all appropriate) X Habitat and Species Research ☐ Biodiversity Management ☐ Student Project ☐ Beyond Quarry Borders 1/3 Abstract The Apollo butterfly (Parnassius apollo Linnaues 1758) is a post-glacial relict, considered extinct from the Romanian fauna by most lepideptorologists.