Flooding Disturbance Structuring Spider and Carabid Beetle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

RYJA V. 2000: Females of Walckenaeria -Species (Araneae, Linyphiidae in the Czech Republic
RŮŽI ČKA V. & BRYJA V. 2000: Females of Walckenaeria -species (Araneae, Linyphiidae in the Czech Republic. Acta Univ. Purkynianae, Biologica 4: 135 S147. Females of the Walckenaeria -species (Araneae, Linyphiidae) in the Czech Republic Vlastimil R ůži čka 1 & Vít ězslav Bryja 2 1Institute of Entomology, Czech Academy of Sciences, Branišovská 31, 370 05 České Bud ějovice, Czech Republic 2Department of Genetics and Molecular Biology, Masaryk University, Kotlá řská 2, 602 00 Brno, Czech Republic Abstract The key to females of nineteen Walckenaeria -species occurring in the Czech Republic is presented. Two nomenclatorical problems within the genus Walckenaeria are solved. Key words: Spiders, Walckenaeria Introduction The genus Walckenaeria now includes the old genera Walckenaeria , Wideria , Cornicularia , Prosopotheca , Trachynella and Tigellinus (see WUNDERLICH 1972). There are nineteen Czech species now included in this genus. Eighteen of them are included in the key (M ILLER 1971) and in the check- list (B UCHAR et al. 1995) of the Czech arachnofauna. W. alticeps was distinguished in the material determined formerly as W. antica according to WUNDERLICH (1972) and KRONESTEDT (1980) during last years. On the other hand, W. simplex is not included in the key of Central European species (H EIMER & NENTWIG 1991). Several additional problems and forgotten facts exist. Results and discussion The figure of the epigyne of W. cuspidata presented in the key of Central European Spiders (H EIMER & NENTWIG 1991) is redrawn from the book of WIEHLE (1960) and is untypical (W UNDERLICH 1972). It was discovered by WUNDERLICH (1970) that the male known as Wideria polita (S IMON , 1881) is in fact the male of W. -

Coleoptera Carabidae) in the Ramsar Wetland: Dayet El Ferd, Tlemcen, Algeria
Biodiversity Journal , 2016, 7 (3): 301–310 Diversity of Ground Beetles (Coleoptera Carabidae) in the Ramsar wetland: Dayet El Ferd, Tlemcen, Algeria Redouane Matallah 1,* , Karima Abdellaoui-hassaine 1, Philippe Ponel 2 & Samira Boukli-hacene 1 1Laboratory of Valorisation of human actions for the protection of the environment and application in public health. University of Tlemcen, BP119 13000 Algeria 2IMBE, CNRS, IRD, Aix-Marseille University, France *Corresponding author: [email protected] ABSTRACT A study on diversity of ground beetle communities (Coleoptera Carabidae) was conducted between March 2011 and February 2012 in the temporary pond: Dayet El Ferd (listed as a Ramsar site in 2004) located in a steppe area on the northwest of Algeria. The samples were collected bimonthly at 6 sampling plots and the gathered Carabidae were identified and coun - ted. A total of 55 species belonging to 32 genera of 7 subfamilies were identified from 2893 collected ground beetles. The most species rich subfamilies were Harpalinae (35 species, 64%) and Trechinae (14 species, 25.45%), others represented by one or two species. Accord- ing to the total individual numbers, Cicindelinae was the most abundant subfamily compris- ing 38.81% of the whole beetles, followed by 998 Harpalinae (34.49%), and 735 Trechinae (25.4%), respectively. The dominant species was Calomera lunulata (Fabricius, 1781) (1087 individuals, 37.57%) and the subdominant species was Pogonus chalceus viridanus (Dejean, 1828) (576 individuals, 19.91%). KEY WORDS Algeria; Carabidae; Diversity; Ramsar wetland “Dayet El Ferd”. Received 28.06.2016; accepted 31.07.2016; printed 30.09.2016 INTRODUCTION gards to vegetation and especially fauna, in partic- ular arthropods. -

Green-Tree Retention and Controlled Burning in Restoration and Conservation of Beetle Diversity in Boreal Forests
Dissertationes Forestales 21 Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests Esko Hyvärinen Faculty of Forestry University of Joensuu Academic dissertation To be presented, with the permission of the Faculty of Forestry of the University of Joensuu, for public criticism in auditorium C2 of the University of Joensuu, Yliopistonkatu 4, Joensuu, on 9th June 2006, at 12 o’clock noon. 2 Title: Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests Author: Esko Hyvärinen Dissertationes Forestales 21 Supervisors: Prof. Jari Kouki, Faculty of Forestry, University of Joensuu, Finland Docent Petri Martikainen, Faculty of Forestry, University of Joensuu, Finland Pre-examiners: Docent Jyrki Muona, Finnish Museum of Natural History, Zoological Museum, University of Helsinki, Helsinki, Finland Docent Tomas Roslin, Department of Biological and Environmental Sciences, Division of Population Biology, University of Helsinki, Helsinki, Finland Opponent: Prof. Bengt Gunnar Jonsson, Department of Natural Sciences, Mid Sweden University, Sundsvall, Sweden ISSN 1795-7389 ISBN-13: 978-951-651-130-9 (PDF) ISBN-10: 951-651-130-9 (PDF) Paper copy printed: Joensuun yliopistopaino, 2006 Publishers: The Finnish Society of Forest Science Finnish Forest Research Institute Faculty of Agriculture and Forestry of the University of Helsinki Faculty of Forestry of the University of Joensuu Editorial Office: The Finnish Society of Forest Science Unioninkatu 40A, 00170 Helsinki, Finland http://www.metla.fi/dissertationes 3 Hyvärinen, Esko 2006. Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests. University of Joensuu, Faculty of Forestry. ABSTRACT The main aim of this thesis was to demonstrate the effects of green-tree retention and controlled burning on beetles (Coleoptera) in order to provide information applicable to the restoration and conservation of beetle species diversity in boreal forests. -

Arachnida, Araneae) Inventory of Hankoniemi, Finland
Biodiversity Data Journal 5: e21010 doi: 10.3897/BDJ.5.e21010 Data Paper Standardized spider (Arachnida, Araneae) inventory of Hankoniemi, Finland Pedro Cardoso‡,§, Lea Heikkinen |, Joel Jalkanen¶, Minna Kohonen|, Matti Leponiemi|, Laura Mattila ¶, Joni Ollonen|, Jukka-Pekka Ranki|, Anni Virolainen |, Xuan Zhou|, Timo Pajunen ‡ ‡ Finnish Museum of Natural History, University of Helsinki, Helsinki, Finland § IUCN SSC Spider & Scorpion Specialist Group, Helsinki, Finland | Department of Biosciences, University of Helsinki, Helsinki, Finland ¶ Department of Environmental Sciences, University of Helsinki, Helsinki, Finland Corresponding author: Pedro Cardoso (pedro.cardoso@helsinki.fi) Academic editor: Jeremy Miller Received: 15 Sep 2017 | Accepted: 14 Dec 2017 | Published: 18 Dec 2017 Citation: Cardoso P, Heikkinen L, Jalkanen J, Kohonen M, Leponiemi M, Mattila L, Ollonen J, Ranki J, Virolainen A, Zhou X, Pajunen T (2017) Standardized spider (Arachnida, Araneae) inventory of Hankoniemi, Finland. Biodiversity Data Journal 5: e21010. https://doi.org/10.3897/BDJ.5.e21010 Abstract Background During a field course on spider taxonomy and ecology at the University of Helsinki, the authors had the opportunity to sample four plots with a dual objective of both teaching on field methods, spider identification and behaviour and uncovering the spider diversity patterns found in the southern coastal forests of Hankoniemi, Finland. As an ultimate goal, this field course intended to contribute to a global project that intends to uncover spider diversity patterns worldwide. With that purpose, a set of standardised methods and procedures was followed that allow the comparability of obtained data with numerous other projects being conducted across all continents. New information A total of 104 species and 1997 adults was collected. -
A New Species of Bembidion Latrielle 1802 from the Ozarks, with a Review
A peer-reviewed open-access journal ZooKeys 147: 261–275 (2011)A new species of Bembidion Latrielle 1802 from the Ozarks... 261 doi: 10.3897/zookeys.147.1872 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research A new species of Bembidion Latrielle 1802 from the Ozarks, with a review of the North American species of subgenus Trichoplataphus Netolitzky 1914 (Coleoptera, Carabidae, Bembidiini) Drew A. Hildebrandt1,†, David R. Maddison2,‡ 1 710 Laney Road, Clinton, MS 39056 USA 2 Department of Zoology, Oregon State University, Corvallis, OR 97331, USA † urn:lsid:zoobank.org:author:038776CA-F70A-4744-96D6-B9B43FB56BB4 ‡ urn:lsid:zoobank.org:author:075A5E9B-5581-457D-8D2F-0B5834CDE04D Corresponding author: David R. Maddison ([email protected]) Academic editor: T. Erwin | Received 31 July 2011 | Accepted 25 August 2011 | Published 16 November 2011 urn:lsid:zoobank.org:pub:52038529-10EA-41A8-BE4F-6B495B610900 Citation: Hildebrandt DA, Maddison DR (2011) A new species of Bembidion Latrielle 1802 from the Ozarks, with a review of the North American species of subgenus Trichoplataphus Netolitzky 1914 (Coleoptera, Carabidae, Bembidiini). In: Erwin T (Ed) Proceedings of a symposium honoring the careers of Ross and Joyce Bell and their contributions to scientific work. Burlington, Vermont, 12–15 June 2010. ZooKeys 147: 261–275. doi: 10.3897/zookeys.147.1872 Abstract A new species of Bembidion (Trichoplataphus Netolitzky) from the Ozark Plateau of Missouri and Arkan- sas is described (Bembidion ozarkense Maddison and Hildebrandt). It is distinguishable from the closely related species, B. rolandi Fall, by characteristics of the male genitalia, and sequences of the genes cyto- chrome oxidase I and 28S ribosomal DNA. -

Coleoptera: Carabidae) Assemblages in a North American Sub-Boreal Forest
Forest Ecology and Management 256 (2008) 1104–1123 Contents lists available at ScienceDirect Forest Ecology and Management journal homepage: www.elsevier.com/locate/foreco Catastrophic windstorm and fuel-reduction treatments alter ground beetle (Coleoptera: Carabidae) assemblages in a North American sub-boreal forest Kamal J.K. Gandhi a,b,1, Daniel W. Gilmore b,2, Steven A. Katovich c, William J. Mattson d, John C. Zasada e,3, Steven J. Seybold a,b,* a Department of Entomology, 219 Hodson Hall, 1980 Folwell Avenue, University of Minnesota, St. Paul, MN 55108, USA b Department of Forest Resources, 115 Green Hall, University of Minnesota, St. Paul, MN 55108, USA c USDA Forest Service, State and Private Forestry, 1992 Folwell Avenue, St. Paul, MN 55108, USA d USDA Forest Service, Northern Research Station, Forestry Sciences Laboratory, 5985 Hwy K, Rhinelander, WI 54501, USA e USDA Forest Service, Northern Research Station, 1831 Hwy 169E, Grand Rapids, MN 55744, USA ARTICLE INFO ABSTRACT Article history: We studied the short-term effects of a catastrophic windstorm and subsequent salvage-logging and Received 9 September 2007 prescribed-burning fuel-reduction treatments on ground beetle (Coleoptera: Carabidae) assemblages in a Received in revised form 8 June 2008 sub-borealforestinnortheasternMinnesota,USA. During2000–2003, 29,873groundbeetlesrepresentedby Accepted 9 June 2008 71 species were caught in unbaited and baited pitfall traps in aspen/birch/conifer (ABC) and jack pine (JP) cover types. At the family level, both land-area treatment and cover type had significant effects on ground Keywords: beetle trap catches, but there were no effects of pinenes and ethanol as baits. -

The Beetle Fauna of Dominica, Lesser Antilles (Insecta: Coleoptera): Diversity and Distribution
INSECTA MUNDI, Vol. 20, No. 3-4, September-December, 2006 165 The beetle fauna of Dominica, Lesser Antilles (Insecta: Coleoptera): Diversity and distribution Stewart B. Peck Department of Biology, Carleton University, 1125 Colonel By Drive, Ottawa, Ontario K1S 5B6, Canada stewart_peck@carleton. ca Abstract. The beetle fauna of the island of Dominica is summarized. It is presently known to contain 269 genera, and 361 species (in 42 families), of which 347 are named at a species level. Of these, 62 species are endemic to the island. The other naturally occurring species number 262, and another 23 species are of such wide distribution that they have probably been accidentally introduced and distributed, at least in part, by human activities. Undoubtedly, the actual numbers of species on Dominica are many times higher than now reported. This highlights the poor level of knowledge of the beetles of Dominica and the Lesser Antilles in general. Of the species known to occur elsewhere, the largest numbers are shared with neighboring Guadeloupe (201), and then with South America (126), Puerto Rico (113), Cuba (107), and Mexico-Central America (108). The Antillean island chain probably represents the main avenue of natural overwater dispersal via intermediate stepping-stone islands. The distributional patterns of the species shared with Dominica and elsewhere in the Caribbean suggest stages in a dynamic taxon cycle of species origin, range expansion, distribution contraction, and re-speciation. Introduction windward (eastern) side (with an average of 250 mm of rain annually). Rainfall is heavy and varies season- The islands of the West Indies are increasingly ally, with the dry season from mid-January to mid- recognized as a hotspot for species biodiversity June and the rainy season from mid-June to mid- (Myers et al. -

DNA Metabarcoding Diet Analysis Reveals Dynamic Feeding Behaviour and Biological Control Potential of Carabid Farmland Co
bioRxiv preprint doi: https://doi.org/10.1101/332312; this version posted May 27, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Title: DNA metabarcoding diet analysis reveals dynamic feeding behaviour and biological 2 control potential of carabid farmland communities 3 4 5 STEFANIYA KAMENOVA1,2,3, VINCENT BRETAGNOLLE2, MANUEL 6 PLANTEGENEST1 & ELSA CANARD1 7 8 1UMR 1349 IGEPP, INRA, Université de Rennes 1, Agrocampus Ouest Rennes, Domaine de 9 la Motte, 35650 Le Rheu, France 10 2Centre d'Etudes Biologiques de Chizé, 79360 Villiers-en-Bois, France 11 3CEES, University of Oslo, 1066 Blindern, 0316 Oslo, Norway 12 13 Corresponding author: Stefaniya Kamenova 14 CEES, University of Oslo, 1066 Blindern, 0316 Oslo, Norway 15 [email protected] 16 17 18 Key-words: DNA metabarcoding diet analysis, carabid beetles, agroecosystems, biological 19 control, food webs 20 21 Running title: Dynamic feeding behaviour of carabid beetles revealed by DNA 22 metabarcoding 23 24 25 Abstract 26 27 Maximizing the delivery of key ecosystem services such as biological control through the 28 management of natural enemy communities is one of the major challenges for modern 29 agriculture. The main obstacle lies in our yet limited capacity of identifying the factors that 30 drive the dynamics of trophic interactions within multi-species assemblages. Invertebrate 31 generalist predators like carabid beetles are known for their dynamic feeding behaviour. -
A DNA Barcode Library for Ground Beetles (Insecta, Coleoptera, Carabidae) of Germany: the Genus Bembidion Latreille, 1802 and Allied Taxa
A peer-reviewed open-access journal ZooKeys A592: DNA 121–141 barcode (2016) library for ground beetles (Insecta, Coleoptera, Carabidae) of Germany... 121 doi: 10.3897/zookeys.592.8316 RESEARCH ARTICLE http://zookeys.pensoft.net Launched to accelerate biodiversity research A DNA barcode library for ground beetles (Insecta, Coleoptera, Carabidae) of Germany: The genus Bembidion Latreille, 1802 and allied taxa Michael J. Raupach1, Karsten Hannig2, Jérome Morinière3, Lars Hendrich4 1 Molecular Taxonomy of Marine Organisms, German Centre of Marine Biodiversity Research (DZMB), Senckenberg am Meer, Südstrand 44, 26382 Wilhelmshaven, Germany 2 Bismarckstraße 5, 45731 Waltrop, Germany 3 Taxonomic coordinator – Barcoding Fauna Bavarica, Bavarian State Collection of Zoology (SNSB – ZSM), Münchhausenstraße 21, 81247 München, Germany 4 Sektion Insecta varia, Bavarian State Collec- tion of Zoology (SNSB – ZSM), Münchhausenstraße 21, 81247 München, Germany Corresponding author: Michael J. Raupach ([email protected]) Academic editor: B. Guéorguiev | Received 1 March 2016 | Accepted 23 April 2016 | Published 25 May 2016 http://zoobank.org/767B2671-3C2A-42B9-9E37-D3B4CF309C94 Citation: Raupach MJ, Hannig K, Morinière J, Hendrich L (2016) A DNA barcode library for ground beetles (Insecta, Coleoptera, Carabidae) of Germany: The genusBembidion Latreille, 1802 and allied taxa. ZooKeys 592: 121–141. doi: 10.3897/zookeys.592.8316 Abstract As molecular identification method, DNA barcoding based on partial cytochromec oxidase subunit 1 (COI) sequences has been proven to be a useful tool for species determination in many insect taxa includ- ing ground beetles. In this study we tested the effectiveness of DNA barcodes to discriminate species of the ground beetle genus Bembidion and some closely related taxa of Germany. -

Los Coleópteros Carábidos De Las Islas Baleares (Coleoptera: Carabidae)
ARTÍCULO DE INVESTIGACIÓN Los coleópteros carábidos de las islas Baleares (Coleoptera: Carabidae) Ground beetle fauna of the Balearic Archipelago JOSÉ SERRANO1, JUAN JOSÉ GUERRERO1, BÁRBARA FERNÁNDEZ1, CARLOS RUIZ2 Y EDUARD PETITPIERRE3 1. Departamento de Zoología y Antropología Física. Universidad de Murcia. Campus de Espinardo, Facultad de Veterinaria. 30071 Murcia (España). 2. Departamento de Ciencias Naturales, Universidad Técnica Particular de Loja, San Cayetano Alto, s/n. 11 01 608 Loja (Ecuador). 3. Departament de Genètica, Universitat des Illes Balears. Mallorca (España). Recibido: 19-09-14. Aceptado: 10-01-2015. Publicado online 17-02-2015. ISSN: 0210-8984 ABSTRACT It has been actualized the inventory of species of the family Carabidae occurring in the Balearic Islands, by joining records of samplings carried out by the Department of Zoology of Murcia, the collection of Prof. Eduard Petitpierre, and the collection of the IMEDEA. Total number of species for the Archipelago is 228; Majorca with Cabrera harbor 190 species, while Minorca harbors 141, and 87 have been recorded in Ibiza and Formentera. It is corroborated the faunistic similarity of islands making up the Gimnesias Archipelago, Majorca and Minorca, whose settlement has been influenced by historical, geographic and climatic factors. The occurrence of lineages as Carabus, Reicheia and Percus¸ suggests a common history with other major islands of the West Mediterranean Basin. Minorca shows a number of peculiarities, namely high species diversity, a low percentage of Mediterranean elements and a high proportion of Asiatic and European elements, perhaps due to a milder climate that favors the occurrence of hygrophylic ground beetles. On the other hand, the Pitiusas show a lower number of species than that theoretically expected according to island area, but to date there is no satisfactory explanation for this finding. -
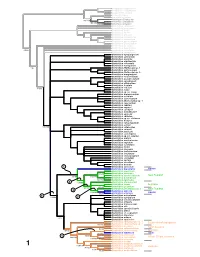
With Description of Bembidion Tahitiense, Sp. Nov. From
Asaphidion yukonense Asaphidion alaskanum Asaphidion curtum Lionepha erasa Lionepha osculans 99/100 Amerizus (Tiruka) sp. 100/100 Amerizus spectabilis 100/100 Amerizus wingatei Bembidion chalceum Bembidion properans Bembidion horni Bembidion hastii Bembidion planum Bembidion transversale Bembidion tetracolum Bembidion genei illigeri Bembidion geniculatum Bembidion cf. csikii Bembidion biguttatum 9 5/92 Bembidion wickhami Bembidion variegatum Bembidion ephippigerum Bembidion salinarium Bembidion assimile Bembidion nigrivestris Bembidion roosevelti Bembidion transparens 7 5/- Bembidion (Nothocys) sp. 1 Bembidion anthracinum Bembidion (Nothocys) sp. 2 Bembidion marginatum Bembidion fortestriatum Bembidion pseudocautum Bembidion canadianum Bembidion elizabethae Bembidion frontale 7 8/73 Bembidion siticum Bembidion lonae Bembidion sp. nr. lonae Bembidion melanopodum Bembidion scitulum Bembidion sexfoveatum Bembidion (Notholopha) sp. 1 Bembidion rugosellum Bembidion rawlinsi Bembidion rogersi Bembidion caoduroi Bembidion mandibulare Bembidion spinolai Bembidion chilense Bembidion sp. nr. chilense Bembidion hirtipes Bembidion rufoplagiatum Bembidion solieri 100/100 Bembidion cillenoides Bembidion calverti Bembidion posticale Bembidion convergens Bembidion sp. nr. ugartei Bembidion aratum Bembidion nubiculosum Bembidion rapidum Bembidion scintillans Bembidion flohri Bembidion idoneum Bembidion indistinctum Bembidion insulatum Bembidion obtusangulum Bembidion cordatum Bembidion varium Bembidion oberthueri Bembidion dorsale 5 Bembidion versutum -

Laboulbeniales (Ascomycota) of the Canary Islands
VIERAEA Vol. 32 107-115 Santa Cruz de Tenerife, diciembre 2004 ISSN 0210-945X Laboulbeniales (Ascomycota) of the Canary Islands ERIK ARNDT * & SERGI SANTAMARÍA** *Anhalt University of Applied Sciences, Department LOEL, Strenzfelder Allee 28, D-06406 Bernburg, Germany. Correspondence address. *Unitat de Botànica, Departament de Biologia Animal, de Biologia Vegetal i d’Ecologia, Facultat de Ciències Universitat Autònoma de Barcelona, E-08193-Bellaterra (Barcelona), SPAIN ARNDT, E. & S. SANTAMARÍA (2004). Laboulbeniales (Ascomycota) de las islas Canarias. VIERAEA 32: 107-115. RESUMEN: Se presenta una lista con 23 especies y 8 géneros de Laboulbeniales (Ascomycota). Seis de ellas son primeras citas para las islas Canarias. La mayoría de las especies citadas infectan Carabidae (Coleoptera). Muchas de las especies de hospedantes (23) son endémicas, siendo sólo nueve las de hospedantes que también se encuentran fuera de la región Macaronésica. Los Carabidae, como familia más diversa de hospedantes, incluyen 21 especies endémicas y 6 de amplia distribución. Las pautas de distribución de Laboulbeniales dependen de dos factores ecológicos (humedad y biodiversidad de los hospedantes) que varían mucho entre las distintas islas. Palabras clave: Laboulbeniales, Carabidae, Staphylinidae, Anthicidae, Blattaria, Ephydridae, islas Canarias. ABSTRACT: The list includes 23 species of Laboulbeniales (Ascomycota) in 8 genera. Six species are new for the Canary Islands. The majority of recorded species infests Carabidae (Coleoptera). Most host species (23) are endemic, only nine hosts also occur outside the Macaronesian region. There are 21 endemic and 6 widespread host species in the Carabidae as most diverse host family. The distribution pattern of Laboulbeniales corresponds with two ecological factors (moisture and host biodiversity respectively) which vary strongly between the different islands.