MDH Amended Directive and Order Regarding Nursing Home Matters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
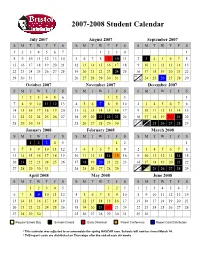
Approved Student Calendar
2007-2008 Student Calendar July 2007 August 2007 September 2007 SMTWT F S SMTWT F S SMTWT F S 1234567 1234 1 8910111213145678910 11 2 3 45678 15 16 17 18 19 20 21 12 13 14 15 16 17 18 9 10 11 12 13 14 15 22 23 24 25 26 27 28 19 20 21 22 23 24 25 16 17 18 19 20 21 22 23 29 30 31 26 27 28 29 30 31 30 24 25 26 27 28 29 October 2007 November 2007 December 2007 SMTWT F S SMTWT F S SMTWT F S 123456 123 1 7891011 12 134567 89102345678 14 15 16 17 18 19 20 11 12 13 14 15 16 17 9 10 11 12 13 14 15 21 22 23 24 25 26 27 18 19 20 21 22 23 24 16 17 18 19 20 21 22 23 24 28 29 30 31 25 26 27 28 29 30 30 31 25 26 27 28 29 January 2008 February 2008 March 2008 SMTWT F S SMTWT F S SMTWT F S 12345 12 1 67891011123456789 2345678 13 14 15 16 17 18 19 10 11 12 13 14 1516 9 1011121314 15 20 21 22 23 24 25 26 17 18 19 20 21 22 23 16 17 18 19 20 21 22 23 24 27 28 29 30 31 24 25 26 27 28 29 30 31 25 26 27 28 29 April 2008 May 2008 June 2008 SMTWT F S SMTWT F S SMTWT F S 12345 123 1234567 6789 10111245678910891011121314 13 14 15 16 17 18 19 11 12 13 14 15 16 17 15 16 17 18 19 20 21 20 21 22 23 24 25 26 18 19 20 21 22 23 24 22 23 24 25 26 27 28 27 28 29 30 25 26 27 28 29 30 31 29 30 Regular School Day Schools Closed Early Dismissal Parent Conference Report Card Distribution * This calendar was adjusted to accommodate the spring NASCAR race. -
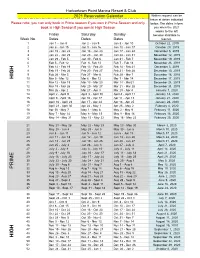
2021 Calandar
Harbortown Point Marina Resort & Club 2021 Reservation Calendar Written request can be taken at dates indicated Please note: you can only book in Prime season if you own in Prime Season and only below. The dates inform book in High Season if you own in High Season you when the 2021 weeks to the left Friday Saturday Sunday become abailable to Week No. Dates Dates Dates reserve. 1 Jan 1 - Jan 8 Jan 2 - Jan 9 Jan 3 - Jan 10 October 22, 2019 2 Jan 8 - Jan 15 Jan 9 - Jan 16 Jan 10 - Jan 17 October 29, 2019 3 Jan 15 - Jan 22 Jan 16 - Jan 23 Jan 17 - Jan 24 November 5, 2019 4 Jan 22 - Jan 29 Jan 23 - Jan 30 Jan 24 - Jan 31 November 12, 2019 5 Jan 29 - Feb 5 Jan 30 - Feb 6 Jan 31 - Feb 7 November 19, 2019 6 Feb 5 - Feb 12 Feb 6- Feb 13 Feb 7 - Feb 14 November 26, 2019 7 Feb 12 - Feb 19 Feb 13 - Feb 20 Feb 14 - Feb 21 December 3, 2019 8 Feb 19 - Feb 26 Feb 20 - Feb 27 Feb 21 - Feb 28 December 10, 2019 9 Feb 26 - Mar 5 Feb 27 - Mar 6 Feb 28 - Mar 7 December 18, 2018 HIGH 10 Mar 5 - Mar 12 Mar 6 - Mar 13 Mar 7 - Mar 14 December 17, 2019 11 Mar 12 - Mar 19 Mar 13 - Mar 20 Mar 14 - Mar21 December 24, 2019 12 Mar 19 - Mar 26 Mar 20 - Mar 27 Mar 21 - Mar 28 December 31, 2019 13 Mar 26 - Apr 2 Mar 27 - Apr 3 Mar 28 - Apr 4 January 7, 2020 14 April 2 - April 9 April 3 - April 10 April 4 - April 11 January 14, 2020 15 April 9 - April 16 Apr 10 - Apr 17 Apr 11 - Apr 18 January 21, 2020 16 April 16 - April 23 Apr 17 - Apr 24 Apr 18 - Apr 25 January 28, 2020 17 April 23 - April 30 Apr 24 - May 1 Apr 25 - May 2 February 4, 2020 18 Apr 30 - May 7 May 1 - May -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

Monday, April 5, 2021
Monday, April 5, 2021 WHERE WE ARE TODAY 3 April 5, 2021 OUR PROGRESS ON VACCINATIONS 4 # DC residents partially or fully vaccinated January 1 5,846 February 1 40,839 March 1 74,811 April 1 162,669 (Apr 2) Data source: DC Health, using received data through 4/2/2021. Data subject to change. April 5, 2021 5 We have more work to do to get all residents vaccinated. Make sure you’re signed up to get your COVID-19 shot. Go to: Call: vaccinate.dc.gov 1-855-363-0333 April 5, 2021 PRE-REGISTRATION SNAPSHOT 6 Approximately 190,621 people have pre-registered for a vaccination appointment and are awaiting an appointment. April 5, 2021 7 If you pre-registered for a vaccination appointment but already received your vaccine elsewhere you can email [email protected] to remove yourself from the pre-registration list. April 5, 2021 NEW AND UPCOMING CLINICS 8 This week, the vaccine clinic at the Convention Center will expand through a partnership with Safeway. Appointment invitations will be sent on April 6. On Friday, April 9, a new high-capacity vaccination site will open at Arena Stage. The site will be operated in partnership with DC Health and MedStar Health. Appointments will be made through vaccinate.dc.gov and the District’s call center. April 5, 2021 THE PATH FORWARD FIVE FACTORS DETERMINING AN ACTIVITY’S RE-OPEN POTENTIAL 10 1. MASKS - Can and is the activity completed with or without wearing a mask? Highest Risk Unmasked, Indoors, Long Time, Very Dense 2. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

2019-2022 Calendar of Major Jewish Holidays
2019-2022 CALENDAR OF MAJOR JEWISH HOLIDAYS Please note: Jewish students may not be able to participate in school activities that take place on the days marked with an *. 2019 2020 2021 2022 PURIM Celebrates the defeat of the plot to destroy March 21 March 10 February 26 March 17 the Jews of Persia. PASSOVER Deliverance of the Jewish people from Egypt. The first *Eve. of April 19 *Eve. of April 8 *Eve. of March 27 *Eve of April 15 and last two days are observed as full holidays. There are *April 20 *April 9 *March 28 *April 16 dietary restrictions against leavened products (such as *April 21 *April 10 *March 29 *April17 bread, pastries, pasta, certain legumes and more) during *April 26 *April 15 *April 3 *April 21 all eight days of the holiday. *April 27 *April 16 *April 4 *April 22 SHAVUOT *Eve. of June 8 *Eve. of May 28 *Eve. of May 16 *Eve of June 3 Feast of Weeks, marks the giving of the Law (Torah) *June 9 *May 29 *May 17 *June 4 at Mt. Sinai. (Often linked with the Confirmation *June 10 *May 30 *May 18 *June 5 of teenagers.) ROSH HASHANAH *Eve. of Sept. 29 *Eve. of Sept. 18 *Eve. of Sept. 6 *Eve of Sept 25 The Jewish New Year; start of the Ten Days of Penitence. *Sept. 30 *Sept. 19 *Sept. 7 *Sept. 26 The first two days are observed as full holidays. *Oct. 1 *Sept. 20 *Sept. 8 *Sept. 27 YOM KIPPUR Day of Atonement; the most solemn day *Eve. -
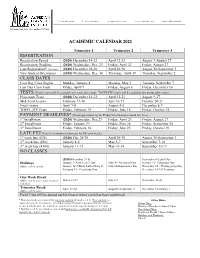
Academic Calendar 2021 Registration Class Dates
T: 214.352.0582 F: 214.350.4119 [email protected] www.alidallas.edu DAL214F01280000 1505 Luna Road, Suite 150, Carrollton, TX 75006 ACADEMIC CALENDAR 2021 Trimester 1 Trimester 2 Trimester 3 REGISTRATION Registration Period (2020) December 14-23 April 12-23 August 9-August 27 Registration Deadline (2020) Wednesday, Dec. 23 Friday, April 23 Friday, August 27 Late Registration* (late fees) (2020) December 28-30 April 26-30 August 30-September 3 New Student Orientation (2020) Wednesday, Dec. 30 Thursday, April 29 Thursday, September 2 CLASS DATES First Day Class Begins Monday, January 4 Monday, May 3 Tuesday, September 7 Last Day Class Ends Friday, April 9 Friday, August 6 Friday, December 10 TESTS (Exam scores will be available one week after exam. TOEFL-ITP scores will be available two weeks after exam.) Placement Tests (2020) December 14-23 April 12-23 August 9-27 Mid-Term Exams February 17-18 June 16-17 October 20-21 Final Exams April 7-8 August 4-5 December 8-9 TOEFL-ITP Exam Friday, February 19 Friday, June 18 Friday, October 22 PAYMENT DEADLINES* (Please pay tuition by the Friday listed below to avoid late fees.) 1st Installment (2020) Wednesday, Dec.23 Friday, April 23 Friday, August 27 2nd Installment Friday, January 29 Friday, May 28 Friday, September 24 3rd Installment Friday, February 26 Friday, June 25 Friday, October 29 LATE FEE (Late fees continue to increase by $50 each week.) 1st week late ($25) (2020) Dec 28-30 April 26-30 August 30-September 3 2nd week late ($50) January 4-8 May 3-7 September 7-10 3rd week late ($100) January 11-15 May 10-14 September 13-17 NO CLASSES (2020) December 31 & September 6-Labor Day Holidays January 1- New Year’s Day May 31- Memorial Day October 11- Columbus Day (Office will be closed) January 18- Martin Luther King Jr. -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -

Pay Date Calendar
Pay Date Information Select the pay period start date that coincides with your first day of employment. Pay Period Pay Period Begins (Sunday) Pay Period Ends (Saturday) Official Pay Date (Thursday)* 1 January 10, 2016 January 23, 2016 February 4, 2016 2 January 24, 2016 February 6, 2016 February 18, 2016 3 February 7, 2016 February 20, 2016 March 3, 2016 4 February 21, 2016 March 5, 2016 March 17, 2016 5 March 6, 2016 March 19, 2016 March 31, 2016 6 March 20, 2016 April 2, 2016 April 14, 2016 7 April 3, 2016 April 16, 2016 April 28, 2016 8 April 17, 2016 April 30, 2016 May 12, 2016 9 May 1, 2016 May 14, 2016 May 26, 2016 10 May 15, 2016 May 28, 2016 June 9, 2016 11 May 29, 2016 June 11, 2016 June 23, 2016 12 June 12, 2016 June 25, 2016 July 7, 2016 13 June 26, 2016 July 9, 2016 July 21, 2016 14 July 10, 2016 July 23, 2016 August 4, 2016 15 July 24, 2016 August 6, 2016 August 18, 2016 16 August 7, 2016 August 20, 2016 September 1, 2016 17 August 21, 2016 September 3, 2016 September 15, 2016 18 September 4, 2016 September 17, 2016 September 29, 2016 19 September 18, 2016 October 1, 2016 October 13, 2016 20 October 2, 2016 October 15, 2016 October 27, 2016 21 October 16, 2016 October 29, 2016 November 10, 2016 22 October 30, 2016 November 12, 2016 November 24, 2016 23 November 13, 2016 November 26, 2016 December 8, 2016 24 November 27, 2016 December 10, 2016 December 22, 2016 25 December 11, 2016 December 24, 2016 January 5, 2017 26 December 25, 2016 January 7, 2017 January 19, 2017 1 January 8, 2017 January 21, 2017 February 2, 2017 2 January -

Caltrans Construction 5-Workday Calendar 2028
CONSTRUCTION 5-WORKDAY CALENDAR Year 2028 JANUARY JULY Sund Mond Tuesd Wedn Thurs Friday Satur Sund Mond Tuesd Wedn Thurs Friday Satur 1 1 678 679 680 681 682 804 805 806 807 2 3 4 5 6 7 8 2 3 4 5 6 7 8 683 684 685 686 687 808 809 810 811 812 9 10 11 12 13 14 15 9 10 11 12 13 14 15 688 689 690 691 813 814 815 816 817 16 17 18 19 20 21 22 16 17 18 19 20 21 22 692 693 694 695 696 818 819 820 821 822 23 24 25 26 27 28 29 23 24 25 26 27 28 29 697 823 30 31 30 31 FEBRUARY AUGUST Sund Mond Tuesd Wedn Thurs Friday Satur Sund Mond Tuesd Wedn Thurs Friday Satur 698 699 700 701 824 825 826 827 1 2 3 4 5 1 2 3 4 5 702 703 704 705 706 828 829 830 831 832 6 7 8 9 10 11 12 6 7 8 9 10 11 12 707 708 709 710 711 833 834 835 836 837 13 14 15 16 17 18 19 13 14 15 16 17 18 19 712 713 714 715 838 839 840 841 842 20 21 22 23 24 25 26 20 21 22 23 24 25 26 716 717 843 844 845 846 27 28 29 27 28 29 30 31 MARCH SEPTEMBER Sund Mond Tuesd Wedn Thurs Friday Satur Sund Mond Tuesd Wedn Thurs Friday Satur 718 719 720 847 1 2 3 4 1 2 721 722 723 724 725 848 849 850 851 5 6 7 8 9 10 11 3 4 5 6 7 8 9 726 727 728 729 730 852 853 854 855 856 12 13 14 15 16 17 18 10 11 12 13 14 15 16 731 732 733 734 735 857 858 859 860 861 19 20 21 22 23 24 25 17 18 19 20 21 22 23 736 737 738 739 862 863 864 865 866 26 27 28 29 30 31 24 25 26 27 28 29 30 APRIL OCTOBER Sund Mond Tuesd Wedn Thurs Friday Satur Sund Mond Tuesd Wedn Thurs Friday Satur 867 868 869 870 871 1 1 2 3 4 5 6 7 740 741 742 743 744 872 873 874 875 2 3 4 5 6 7 8 8 9 10 11 12 13 14 745 746 747 748 749 876 877 878 879 880 -

Pay Schedule
Department of Employee and Retiree Services MONTGOMERY COUNTY PUBLIC SCHOOLS Rockville, Maryland SCHEDULE OF PAY PERIODS AND PAYDAYS FOR ALL MONTGOMERY COUNTY PUBLIC SCHOOLS EMPLOYEES FY 2020–2021 Pay Period Online Attendance Must Be Saturday Thru Friday Pay Period Payday Dates Footnotes Posted and Released By Dates and 4:00 p.m. Numbers June 06-June 19, 2020 Friday, June 19, 2020 Thursday, July 02, 2020 PP01 June 20–July 03, 2020 Monday, July 06, 2020 Friday, July 17, 2020 PP02 July 04–July 17, 2020 Monday, July 20, 2020 Friday, July 31, 2020 PP03 July 18–July 31, 2020 Monday, August 03, 2020 Friday, August 14, 2020 PP04 August 01–August 14, 2020 Monday, August 17, 2020 Friday, August 28, 2020 PP05 August 15–August 28, 2020 Monday, August 31, 2020 Friday, September 11, 2020 PP06 August 29–September 11, 2020 Friday, September 11, 2020 *Friday, September 18 , 2020 PP07 September 12–September 25, 2020 Monday, September 28, 2020 *Friday, October 09, 2020 PP08 September 26–October 09, 2020 Friday, October 09, 2020 *Friday, October 23, 2020 PP09 October 10–October 23, 2020 Monday, October 26, 2020 *Friday, November 06, 2020 PP10 October 24–November 06, 2020 Monday, November 9, 2020 *Friday, November 20, 2020 PP11 November 07–November 20, 2020 Friday, November 20, 2020 *Friday, December 04, 2020 PP12 November 21–December 04, 2020 Monday, December 7, 2020 *Friday, December 18, 2020 PP13 December 05–December 18, 2020 Friday, December 18, 2020 *Thursday, December 31, 2020 PP14 December 19–January 01, 2021 Monday, January 04, 2021 *Friday, January -

Date of Close Contact Exposure
Date of Close Contact Exposure 7 days 10 days 14 days Monday, November 16, 2020 Tuesday, November 24, 2020 Friday, November 27, 2020 Tuesday, December 1, 2020 Tuesday, November 17, 2020 Wednesday, November 25, 2020 Saturday, November 28, 2020 Wednesday, December 2, 2020 Wednesday, November 18, 2020 Thursday, November 26, 2020 Sunday, November 29, 2020 Thursday, December 3, 2020 Thursday, November 19, 2020 Friday, November 27, 2020 Monday, November 30, 2020 Friday, December 4, 2020 Friday, November 20, 2020 Saturday, November 28, 2020 Tuesday, December 1, 2020 Saturday, December 5, 2020 Saturday, November 21, 2020 Sunday, November 29, 2020 Wednesday, December 2, 2020 Sunday, December 6, 2020 Sunday, November 22, 2020 Monday, November 30, 2020 Thursday, December 3, 2020 Monday, December 7, 2020 Monday, November 23, 2020 Tuesday, December 1, 2020 Friday, December 4, 2020 Tuesday, December 8, 2020 Tuesday, November 24, 2020 Wednesday, December 2, 2020 Saturday, December 5, 2020 Wednesday, December 9, 2020 Wednesday, November 25, 2020 Thursday, December 3, 2020 Sunday, December 6, 2020 Thursday, December 10, 2020 Thursday, November 26, 2020 Friday, December 4, 2020 Monday, December 7, 2020 Friday, December 11, 2020 Friday, November 27, 2020 Saturday, December 5, 2020 Tuesday, December 8, 2020 Saturday, December 12, 2020 Saturday, November 28, 2020 Sunday, December 6, 2020 Wednesday, December 9, 2020 Sunday, December 13, 2020 Sunday, November 29, 2020 Monday, December 7, 2020 Thursday, December 10, 2020 Monday, December 14, 2020 Monday, November