A C-Type Lectin Fold Gene from Japanese Scallop Mizuhopecten Yessoensis, Involved with Immunity and Metamorphosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analysis of Synonymous Codon Usage Patterns in Sixty-Four Different Bivalve Species
Analysis of synonymous codon usage patterns in sixty-four diVerent bivalve species Marco Gerdol1, Gianluca De Moro1, Paola Venier2 and Alberto Pallavicini1 1 Department of Life Sciences, University of Trieste, Trieste, Italy 2 Department of Biology, University of Padova, Padova, Italy ABSTRACT Synonymous codon usage bias (CUB) is a defined as the non-random usage of codons encoding the same amino acid across diVerent genomes. This phenomenon is common to all organisms and the real weight of the many factors involved in its shaping still remains to be fully determined. So far, relatively little attention has been put in the analysis of CUB in bivalve mollusks due to the limited genomic data available. Taking advantage of the massive sequence data generated from next generation sequencing projects, we explored codon preferences in 64 diVerent species pertaining to the six major evolutionary lineages in Bivalvia. We detected remarkable diVerences across species, which are only partially dependent on phylogeny. While the intensity of CUB is mild in most organisms, a heterogeneous group of species (including Arcida and Mytilida, among the others) display higher bias and a strong preference for AT-ending codons. We show that the relative strength and direction of mutational bias, selection for translational eYciency and for translational accuracy contribute to the establishment of synonymous codon usage in bivalves. Although many aspects underlying bivalve CUB still remain obscure, we provide for the first time an overview of this phenomenon -

Pectenovarin, a New Ovarian Carotenoprotein from Japanese Scallop Mizuhopecten Yessoensis
molecules Article Pectenovarin, A New Ovarian Carotenoprotein from Japanese Scallop Mizuhopecten yessoensis Satoko Matsunaga 1,* , Hiroki Ikeda 2 and Ryuichi Sakai 2 1 Department of Material and Environmental Engineering, National Institute of Technology HAKODATE College, 14-1 Tokura-cho, Hakodate, Hokkaido 042-8501, Japan 2 Faculty and Graduate School of Fisheries Sciences, Hokkaido University, 3-1-1 Minato-cho, Hakodate 041-8611, Japan; [email protected] (H.I.); ryu.sakai@fish.hokudai.ac.jp (R.S.) * Correspondence: [email protected]; Tel.: +81-138-59-6463 Academic Editor: Valeria Costantino Received: 9 June 2020; Accepted: 30 June 2020; Published: 3 July 2020 Abstract: The scallop Mizuhopecten yessoensis accumulates carotenoids in the ovary during the maturation stage. Its conspicuous pink color implies the presence of carotenoprotein. However, the carotenoprotein from the scallop ovary has never been isolated and characterized, probably due to its instability and complexity. Here, we developed an extraction and isolation procedure for the carotenoprotein by employing a basic buffer containing potassium bromide to facilitate its efficient extraction from the ovary, and we succeeded in obtaining the carotenoprotein, termed pectenovarin. The carotenoid composition of the pectenovarin was similar to that of the ovary. The N-terminal and internal amino acid sequences of pectenovarin showed a high similarity to those of vitellogenin, the precursor of egg yolk protein under analysis. Keywords: carotenoproteins; carotenoids; scallop ovary; Japanese scallop 1. Introduction Carotenoids are one of the essential classes of metabolites in a wide variety of living organisms. More than 750 different carotenoids have been reported to date [1]. It is known that carotenoids bind to proteins, termed carotenoproteins, to stabilize themselves against light or heat or to manipulate their physical properties, including solubility and color. -

The Shell Matrix of the European Thorny Oyster, Spondylus Gaederopus: Microstructural and Molecular Characterization
The shell matrix of the european thorny oyster, Spondylus gaederopus: microstructural and molecular characterization. Jorune Sakalauskaite, Laurent Plasseraud, Jérôme Thomas, Marie Alberic, Mathieu Thoury, Jonathan Perrin, Frédéric Jamme, Cédric Broussard, Beatrice Demarchi, Frédéric Marin To cite this version: Jorune Sakalauskaite, Laurent Plasseraud, Jérôme Thomas, Marie Alberic, Mathieu Thoury, et al.. The shell matrix of the european thorny oyster, Spondylus gaederopus: microstructural and molecular characterization.. Journal of Structural Biology, Elsevier, 2020, 211 (1), pp.107497. 10.1016/j.jsb.2020.107497. hal-02906399 HAL Id: hal-02906399 https://hal.archives-ouvertes.fr/hal-02906399 Submitted on 17 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. The shell matrix of the European thorny oyster, Spondylus gaederopus: microstructural and molecular characterization List of authors: Jorune Sakalauskaite1,2, Laurent Plasseraud3, Jérôme Thomas2, Marie Albéric4, Mathieu Thoury5, Jonathan Perrin6, Frédéric Jamme6, Cédric Broussard7, Beatrice Demarchi1, Frédéric Marin2 Affiliations 1. Department of Life Sciences and Systems Biology, University of Turin, Via Accademia Albertina 13, 10123 Turin, Italy; 2. Biogeosciences, UMR CNRS 6282, University of Burgundy-Franche-Comté, 6 Boulevard Gabriel, 21000 Dijon, France. 3. Institute of Molecular Chemistry, ICMUB UMR CNRS 6302, University of Burgundy- Franche-Comté, 9 Avenue Alain Savary, 21000 Dijon, France. -

Computational Modelling of Wet Adhesive Mussel Foot Proteins (Bivalvia): Insights Into the Evolutionary Convolution in Diverse Perspectives P
www.nature.com/scientificreports OPEN Computational modelling of wet adhesive mussel foot proteins (Bivalvia): Insights into the evolutionary convolution in diverse perspectives P. P. Anand & Y. Shibu Vardhanan Underwater adhesion in mussels (Bivalvia) is an extreme adaptation to achieve robust and frm wet adhesion in the freshwater/brackish/ocean, which biochemically shaped through millions of years. The protein-based adhesion has huge prospective in various felds like industry, medical, etc. Currently, no comprehensive records related to the systematic documentation of structural and functional properties of Mussel foot proteins (Mfps). In this study, we identifed the nine species of bivalves in which the complete sequence of at least one adhesive protein is known. The insilico characterization revealed the specifc physio-chemical structural and functional characters of each Mfps. The evolutionary analyses of selected bivalves are mainly based on Mfps, Mitogenome, and TimeTree. The outcome of the works has great applications for designing biomimetic materials in future. Some groups of mussels are capable to produce proteinaceous glue- like sticky material known as byssus thread made by an array of foot proteins (fps). Tis byssus contains mainly four parts i.e. Plaque, thread, stem, and root. Individual threads proximally merged together to form stem and base of the stem (root) deeply anchored at the base of animal foot. Each byssus threads terminating distally with a fattened plaque which mediates adhesion to the substratum1–4. Each part of the byssus thread complex formed by the auto-assembly of secretory products originating from four distinct glands enclosed in the mussel foot4,5. Tese mussel foot protein (Mfps), mastered the ability to binding the diverse substratum by using adhesive plaques. -

Comparative Large-Scale Mitogenomics Evidences Clade-Specific Evolutionary Trends in Mitochondrial Dnas of Bivalvia
GBE Comparative Large-Scale Mitogenomics Evidences Clade-Specific Evolutionary Trends in Mitochondrial DNAs of Bivalvia Federico Plazzi*, Guglielmo Puccio, and Marco Passamonti Department of Biological, Geological and Environmental Sciences, University of Bologna, via Selmi, 3 – 40126 Bologna, Italy *Corresponding author: E-mail: [email protected]. Accepted: July 31, 2016 Abstract Despite the figure of complete bivalve mitochondrial genomes keeps growing, an assessment of the general features of these genomes in a phylogenetic framework is still lacking, despite the fact that bivalve mitochondrial genomes are unusual under different aspects. In this work, we constructed a dataset of one hundred mitochondrial genomes of bivalves to perform the first systematic comparative mitogenomic analysis, developing a phylogenetic background to scaffold the evolutionary history of the class’ mito- chondrial genomes. Highly conserved domains were identified in all protein coding genes; however, four genes (namely, atp6, nad2, nad4L,andnad6) were found to be very divergent for many respects, notwithstanding the overall purifying selection working on those genomes. Moreover, the atp8 gene was newly annotated in 20 mitochondrial genomes, where it was previously declared as lacking or only signaled. Supernumerary mitochondrial proteins were compared, but it was possible to find homologies only among strictly related species. The rearrangement rate on the molecule is too high to be used as a phylogenetic marker, but here we demonstrate for the first time in mollusks that there is correlation between rearrangement rates and evolutionary rates. We also developed a new index (HERMES) to estimate the amount of mitochondrial evolution. Many genomic features are phylogenetically congruent and this allowed us to highlight three main phases in bivalve history: the origin, the branching of palaeoheterodonts, and the second radiation leading to the present-day biodiversity. -

Genomic Signatures of G-Protein-Coupled Receptor
Genomic signatures of G-protein-coupled royalsocietypublishing.org/journal/rspb receptor expansions reveal functional transitions in the evolution of cephalopod signal transduction Research Elena A. Ritschard1,2, Robert R. Fitak2, Oleg Simakov1 and So¨nke Johnsen2 Cite this article: Ritschard EA, Fitak RR, Simakov O, Johnsen S. 2019 Genomic 1Department of Molecular Evolution and Development, University of Vienna, Vienna, Austria 2Department of Biology, Duke University, Durham, NC, USA signatures of G-protein-coupled receptor expansions reveal functional transitions in the EAR, 0000-0002-4956-9703; RRF, 0000-0002-7398-6259; OS, 0000-0002-3585-4511; SJ, 0000-0002-3943-8320 evolution of cephalopod signal transduction. Proc. R. Soc. B 286: 20182929. Coleoid cephalopods show unique morphological and neural novelties, such http://dx.doi.org/10.1098/rspb.2018.2929 as arms with tactile and chemosensory suckers and a large complex nervous system. The evolution of such cephalopod novelties has been attributed at a genomic level to independent gene family expansions, yet the exact associ- ation and the evolutionary timing remain unclear. In the octopus genome, Received: 26 December 2018 one such expansion occurred in the G-protein-coupled receptors (GPCRs) Accepted: 4 February 2019 repertoire, a superfamily of proteins that mediate signal transduction. Here, we assessed the evolutionary history of this expansion and its relationship with cephalopod novelties. Using phylogenetic analyses, at least two cepha- lopod- and two octopus-specific GPCR expansions were identified. Signatures of positive selection were analysed within the four groups, and Subject Category: the locations of these sequences in the Octopus bimaculoides genome were Genetics and genomics inspected. -

List of Bivalve Molluscs from British Columbia, Canada
List of Bivalve Molluscs from British Columbia, Canada Compiled by Robert G. Forsyth Research Associate, Invertebrate Zoology, Royal BC Museum, 675 Belleville Street, Victoria, BC V8W 9W2; [email protected] Rick M. Harbo Research Associate, Invertebrate Zoology, Royal BC Museum, 675 Belleville Street, Victoria BC V8W 9W2; [email protected] Last revised: 11 October 2013 INTRODUCTION Classification rankings are constantly under debate and review. The higher classification utilized here follows Bieler et al. (2010). Another useful resource is the online World Register of Marine Species (WoRMS; Gofas 2013) where the traditional ranking of Pteriomorphia, Palaeoheterodonta and Heterodonta as subclasses is used. This list includes 237 bivalve species from marine and freshwater habitats of British Columbia, Canada. Marine species (206) are mostly derived from Coan et al. (2000) and Carlton (2007). Freshwater species (31) are from Clarke (1981). Common names of marine bivalves are from Coan et al. (2000), who adopted most names from Turgeon et al. (1998); common names of freshwater species are from Turgeon et al. (1998). Changes to names or additions to the fauna since these two publications are marked with footnotes. Marine groups are in black type, freshwater taxa are in blue. Introduced (non-indigenous) species are marked with an asterisk (*). Marine intertidal species (n=84) are noted with a dagger (†). Quayle (1960) published a BC Provincial Museum handbook, The Intertidal Bivalves of British Columbia. Harbo (1997; 2011) provided illustrations and descriptions of many of the bivalves found in British Columbia, including an identification guide for bivalve siphons and “shows”. Lamb & Hanby (2005) also illustrated many species. -

Japan Update to 05.04.2021 Approval No Name Address Products Number FROZEN CHUM SALMON DRESSED (Oncorhynchus Keta)
Japan Update to 05.04.2021 Approval No Name Address Products Number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta). FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus). FROZEN JAPANESE SARDINE ROUND (Sardinops 81,Misaki-Cho,Rausu- Kaneshin Tsuyama melanostictus). FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma). 1 VN01870001 Cho, Menashi- Co.,Ltd FROZEN ALASKA POLLACK ROUND (Theragra chalcogramma). FROZEN PACIFIC COD Gun,Hokkaido,Japan DRESSED. (Gadus macrocephalus). FROZEN PACIFIC COD ROUND. (Gadus macrocephalus) Maekawa Shouten Hokkaido Nemuro City Fresh Fish (Excluding Fish By-Product); Fresh Bivalve Mollusk.; Frozen Fish (Excluding 2 VN01860002 Co., Ltd Nishihamacho 10-177 Fish By-Product); Frozen Processed Bivalve Mollusk; Frozen Chum Salmon(Round,Dressed,Semi-Dressed,Fillet,Head,Bone,Skin); Frozen 1-35-1 Alaska Pollack(Round,Dressed,Semi-Dressed,Fillet); Frozen Pacific Taiyo Sangyo Co.,Ltd. 3 VN01840003 Showachuo,Kushiro- Cod(Round,Dressed,Semi-Dressed,Fillet); Frozen Pacific Saury(Round,Dressed,Semi- Kushiro Factory City,Hokkaido,Japan Dressed); Frozen Chub Mackerel(Round,Fillet); Frozen Blue Mackerel(Round,Fillet); Frozen Salted Pollack Roe 3-9 Komaba- Taiyo Sangyo Co.,Ltd. 4 VN01860004 Cho,Nemuro- Frozen Fish ; Frozen Processed Fish; (Excluding By-Product) Nemuro Factory City,Hokkaido,Japan 3-2-20 Kitahama- Marutoku Abe Suisan 5 VN01920005 Cho,Monbetu- Frozen Chum Salmon Dressed; Frozen Salmon Dressed Co.,Ltd City,Hokkaido,Japan Frozen Chum Salmon(Round,Semi-Dressed,Fillet); Frozen Salmon Milt; Frozen Pink Salmon(Round,Semi-Dressed,Dressed,Fillet); -
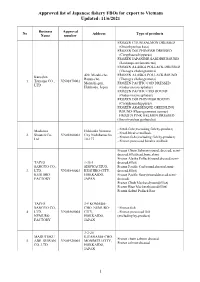
Approved List of Japanese Fishery Fbos for Export to Vietnam Updated: 11/6/2021
Approved list of Japanese fishery FBOs for export to Vietnam Updated: 11/6/2021 Business Approval No Address Type of products Name number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta) FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus) FROZEN JAPANESE SARDINE ROUND (Sardinops melanostictus) FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma) 420, Misaki-cho, FROZEN ALASKA POLLACK ROUND Kaneshin Rausu-cho, (Theragra chalcogramma) 1. Tsuyama CO., VN01870001 Menashi-gun, FROZEN PACIFIC COD DRESSED LTD Hokkaido, Japan (Gadus macrocephalus) FROZEN PACIFIC COD ROUND (Gadus macrocephalus) FROZEN DOLPHIN FISH ROUND (Coryphaena hippurus) FROZEN ARABESQUE GREENLING ROUND (Pleurogrammus azonus) FROZEN PINK SALMON DRESSED (Oncorhynchus gorbuscha) - Fresh fish (excluding fish by-product) Maekawa Hokkaido Nemuro - Fresh bivalve mollusk. 2. Shouten Co., VN01860002 City Nishihamacho - Frozen fish (excluding fish by-product) Ltd 10-177 - Frozen processed bivalve mollusk Frozen Chum Salmon (round, dressed, semi- dressed,fillet,head,bone,skin) Frozen Alaska Pollack(round,dressed,semi- TAIYO 1-35-1 dressed,fillet) SANGYO CO., SHOWACHUO, Frozen Pacific Cod(round,dressed,semi- 3. LTD. VN01840003 KUSHIRO-CITY, dressed,fillet) KUSHIRO HOKKAIDO, Frozen Pacific Saury(round,dressed,semi- FACTORY JAPAN dressed) Frozen Chub Mackerel(round,fillet) Frozen Blue Mackerel(round,fillet) Frozen Salted Pollack Roe TAIYO 3-9 KOMABA- SANGYO CO., CHO, NEMURO- - Frozen fish 4. LTD. VN01860004 CITY, - Frozen processed fish NEMURO HOKKAIDO, (excluding by-product) FACTORY JAPAN -

Molecular Identification of Steroidogenesis-Related Genes in Scallops and Their Potential Roles in Gametogenesis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Molecular identification of steroidogenesis-related genes in scallops and their potential roles in gametogenesis 著者 Tongchai Thitiphuree, Kazue Nagasawa, Makoto Osada journal or The Journal of Steroid Biochemistry and publication title Molecular Biology volume 186 page range 22-23 year 2019-02 URL http://hdl.handle.net/10097/00128371 doi: 10.1016/j.jsbmb.2018.09.004 Creative Commons : 表示 - 非営利 - 改変禁止 http://creativecommons.org/licenses/by-nc-nd/3.0/deed.ja The Journal of Steroid Biochemistry and Molecular Biology, Vol.186 (2019) [DOI: 10.1016/j.jsbmb.2018.09.004] Molecular identification of steroidogenesis-related genes in scallops and their potential roles in gametogenesis Tongchai THITIPHUREE *1, Kazue NAGASAWA *1, Makoto OSADA *1 *1 Laboratory of Aquacultural Biology, Graduate School of Agricultural Science, Tohoku University, 468-1 Aoba, Aramaki, Aoba-ku, Sendai, Miyagi, Japan Abstract Sex steroids are crucial for controlling gametogenesis and germ cell maturation in vertebrates. It has been proposed that Yesso scallop (Mizuhopecten yessoensis) has the same sex steroids as those animals, but the scallop biosynthetic pathway is unclear. In this study, we characterized several steroidogenesis-related genes in M. yessoensis and proposed a putative biosynthetic pathway for sex steroids that is similar to that of vertebrates. Specifically, we identified several steroidogenesis-related gene sequences that encode steroid metabolizing enzymes: StAR-related lipid transfer (START) protein, 17α-hydroxylase, 17,20-lyase (cyp17a), 17β-hydroxysteroid dehydrogenase (hsd17b), and 3β-hydroxysteroid dehydrogenase (hsd3b). We sampled adult scallops throughout their reproductive phase to compare their degree of maturation with their intensity of mRNA expression. -

The Evolution of Extreme Longevity in Modern and Fossil Bivalves
Syracuse University SURFACE Dissertations - ALL SURFACE August 2016 The evolution of extreme longevity in modern and fossil bivalves David Kelton Moss Syracuse University Follow this and additional works at: https://surface.syr.edu/etd Part of the Physical Sciences and Mathematics Commons Recommended Citation Moss, David Kelton, "The evolution of extreme longevity in modern and fossil bivalves" (2016). Dissertations - ALL. 662. https://surface.syr.edu/etd/662 This Dissertation is brought to you for free and open access by the SURFACE at SURFACE. It has been accepted for inclusion in Dissertations - ALL by an authorized administrator of SURFACE. For more information, please contact [email protected]. Abstract: The factors involved in promoting long life are extremely intriguing from a human perspective. In part by confronting our own mortality, we have a desire to understand why some organisms live for centuries and others only a matter of days or weeks. What are the factors involved in promoting long life? Not only are questions of lifespan significant from a human perspective, but they are also important from a paleontological one. Most studies of evolution in the fossil record examine changes in the size and the shape of organisms through time. Size and shape are in part a function of life history parameters like lifespan and growth rate, but so far little work has been done on either in the fossil record. The shells of bivavled mollusks may provide an avenue to do just that. Bivalves, much like trees, record their size at each year of life in their shells. In other words, bivalve shells record not only lifespan, but also growth rate. -

The Gene-Rich Genome of the Scallop Pecten Maximus Nathan J
GigaScience, 9, 2020, 1–13 doi: 10.1093/gigascience/giaa037 Data Note Downloaded from https://academic.oup.com/gigascience/article/9/5/giaa037/5827190 by FMC Corporation Librarian user on 02 June 2021 DATA NOTE The gene-rich genome of the scallop Pecten maximus Nathan J. Kenny1,2, Shane A. McCarthy3, Olga Dudchenko4,5, Katherine James1,6, Emma Betteridge7,CraigCorton7, Jale Dolucan7,8, Dan Mead7, Karen Oliver7, Arina D. Omer4, Sarah Pelan7, Yan Ryan9,10, Ying Sims7, Jason Skelton7, Michelle Smith7, James Torrance7, David Weisz4, Anil Wipat9, Erez L Aiden4,5,11,12, Kerstin Howe7 and Suzanne T. Williams 1,* 1Natural History Museum, Department of Life Sciences, Cromwell Road, London SW7 5BD, UK; 2Present address: Oxford Brookes University, Headington Road, Oxford OX3 0BP, UK; 3University of Cambridge, Department of Genetics, Cambridge CB2 3EH, UK; 4The Center for Genome Architecture, Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX 77030, USA; 5The Center for Theoretical Biological Physics, Rice University, 6100 Main St, Houston, TX 77005-1827, USA; 6Present address: Department of Applied Sciences, Faculty of Health and Life Sciences, Northumbria University, Newcastle upon Tyne NE1 8ST, UK; 7Wellcome Sanger Institute, Cambridge CB10 1SA, UK; 8Present address: Freeline Therapeutics Limited, Stevenage Bioscience Catalyst, Gunnels Wood Road, Stevenage, Hertfordshire, SG1 2FX, UK; 9School of Computing, Newcastle University, Newcastle upon Tyne NE1 7RU, UK; 10Institute of Infection and Global Health, Liverpool University, iC2, 146 Brownlow Hill, Liverpool L3 5RF, UK; 11Shanghai Institute for Advanced Immunochemical Studies, Shanghai Tech University, Shanghai, China and 12School of Agriculture and Environment, University of Western Australia, Perth, Australia.