Evolution of Atomic Theory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cathode-Ray Tube Displays for Medical Imaging
DIGITAL IMAGING BASICS Cathode-Ray Tube Displays for Medical Imaging Peter A. Keller This paper will discuss the principles of cathode-ray crease the velocity of the electron beam for tube displays in medical imaging and the parameters increased light output from the screen; essential to the selection of displays for specific 4. a focusing section to bring the electron requirements. A discussion of cathode-ray tube fun- beam to a sharp focus at the screen; damentals and medical requirements is included. 9 1990bu W.B. Saunders Company. 5. a deflection system to position the electron beam to a desired location on the screen or KEY WORDS: displays, cathode ray tube, medical scan the beam in a repetitive pattern; and irnaging, high resolution. 6. a phosphor screen to convert the invisible electron beam to visible light. he cathode-ray tube (CRT) is the heart of The assembly of electrodes or elements mounted T almost every medical display and its single within the neck of the CRT is commonly known most costly component. Brightness, resolution, as the "electron gun" (Fig 2). This is a good color, contrast, life, cost, and viewer comfort are analogy, because it is the function of the electron gun to "shoot" a beam of electrons toward the all strongly influenced by the selection of a screen or target. The velocity of the electron particular CRT by the display designer. These beam is a function of the overall accelerating factors are especially important for displays used voltage applied to the tube. For a CRT operating for medical diagnosis in which patient safety and at an accelerating voltage of 20,000 V, the comfort hinge on the ability of the display to electron velocity at the screen is about present easily readable, high-resolution images 250,000,000 mph, or about 37% of the velocity of accurately and rapidly. -

Appendix F. Glossary
Appendix F. Glossary 2DEG 2-dimensional electron gas A/D Analog to digital AAAR American Association for Aerosol Research ADC Analog-digital converter AEM Analytical electron microscopy AFM Atomic force microscope/microscopy AFOSR Air Force Office of Scientific Research AIST (Japan) Agency of Industrial Science and Technology AIST (Japan, MITI) Agency of Industrial Science and Technology AMLCD Active matrix liquid crystal display AMM Amorphous microporous mixed (oxides) AMO Atomic, molecular, and optical AMR Anisotropic magnetoresistance ARO (U.S.) Army Research Office ARPES Angle-resolved photoelectron spectroscopy ASET (Japan) Association of Super-Advanced Electronics Technologies ASTC Australia Science and Technology Council ATP (Japan) Angstrom Technology Partnership ATP Adenosine triphosphate B Magnetic flux density B/H loop Closed figure showing B (magnetic flux density) compared to H (magnetic field strength) in a magnetizable material—also called hysteresis loop bcc Body-centered cubic BMBF (Germany) Ministry of Education, Science, Research, and Technology (formerly called BMFT) BOD-FF Bond-order-dependent force field BRITE/EURAM Basic Research of Industrial Technologies for Europe, European Research on Advanced Materials program CAD Computer-assisted design CAIBE Chemically assisted ion beam etching CBE Chemical beam epitaxy 327 328 Appendix F. Glossary CBED Convergent beam electron diffraction cermet Ceramic/metal composite CIP Cold isostatic press CMOS Complementary metal-oxide semiconductor CMP Chemical mechanical polishing -

Chem 103, Section F0F Unit I
Lecture 4 - Observations that Led to the Chem 103, Section F0F Nuclear Model of the Atom Unit I - An Overview of Chemistry Dalton’s theory proposed that atoms were indivisible particles. Lecture 4 • By the late 19th century, this aspect of Dalton’s theory was being challenged. • Work with electricity lead to the discovery of the electron, • Some observations that led to the nuclear model as a particle that carried a negative charge. for the structure of the atom • The modern view of the atomic structure and the elements • Arranging the elements into a (periodic) table 2 Lecture 4 - Observations that Led to the Lecture 4 - Observations that Led to the Nuclear Model of the Atom Nuclear Model of the Atom The cathode ray In 1897, J.J. Thomson (1856-1940) studies how cathode rays • Cathode rays were shown to be electrons are affected by electric and magnetic fields • This allowed him to determine the mass/charge ration of an electron Cathode rays are released by metals at the cathode 3 4 Lecture 4 - Observations that Led to the Lecture 4 - Observations that Led to the Nuclear Model of the Atom Nuclear Model of the Atom In 1897, J.J. Thomson (1856-1940) studies how cathode rays In 1897, J.J. Thomson (1856-1940) studies how cathode rays are affected by electric and magnetic fields are affected by electric and magnetic fields • Thomson estimated that the mass of an electron was less • Thomson received the 1906 Nobel Prize in Physics for his that 1/1000 the mass of the lightest atom, hydrogen!! work. -

Glossary of Scientific Terms in the Mystery of Matter
GLOSSARY OF SCIENTIFIC TERMS IN THE MYSTERY OF MATTER Term Definition Section acid A substance that has a pH of less than 7 and that can react with 1 metals and other substances. air The mixture of oxygen, nitrogen, and other gasses that is consistently 1 present around us. alchemist A person who practices a form of chemistry from the Middle Ages 1 that was concerned with transforming various metals into gold. Alchemy A type of science and philosophy from the Middle Ages that 1 attempted to perform unusual experiments, taking something ordinary and turning it into something extraordinary. alkali metals Any of a group of soft metallic elements that form alkali solutions 3 when they combine with water. They include lithium, sodium, potassium, rubidium, cesium, and francium. alkaline earth Any of a group of metallic elements that includes beryllium, 3 metals magnesium, calcium, strontium, barium, and radium. alpha particle A positively charged particle, indistinguishable from a helium atom 5, 6 nucleus and consisting of two protons and two neutrons. alpha decay A type of radioactive decay in which a nucleus emits 6 an alpha particle. aplastic anemia A disorder of the bone marrow that results in too few blood cells. 4 apothecary The person in a pharmacy who distributes medicine. 1 atom The smallest component of an element that shares the chemical 1, 2, 3, 4, 5, 6 properties of the element and contains a nucleus with neutrons, protons, and electrons. atomic bomb A bomb whose explosive force comes from a chain reaction based on 6 nuclear fission. atomic number The number of protons in the nucleus of an atom. -

Pioneers of Atomic Theory Darius Bermudez Discoverers of the Atom
Pioneers of Atomic Theory Darius Bermudez Discoverers of the Atom Democritus- Greek Philosopher proposed that if something was divided enough times, eventually the particles would be too small to divide any further. Ex: Identify this Greek philosopher who postulated that if an object was divided enough times, there would eventually be small particles that could not be divided any further. Discoverers of the Atom John Dalton- English chemist who made the “billiard ball” atom model. First to prove that rainfall was a result of temperature change. He was the first scientist after Democritus to build on atomic theory. He also created a law on partial pressures. Common Clues: Partial pressures, pioneer of atomic theory, and temperature change causes rainfall. The image cannot be displayed. Your computer may not have enough memory to open the image, or the image may have been corrupted. Restart your computer, and then open the file again. If the red x still appears, you may have to delete the image and then insert it again. The image cannot be displayed. Your computer may not have enough memory to open the image, or the image may have been corrupted. Restart your computer, and then open the file again. If the red x still appears, you may have to delete the image and then insert it again. Discoverers of the Atom J.J. Thomson- English Scientist who discovered electrons through a cathode. Made the “plum pudding model” with Lord Kelvin (Kelvin Scale) which stated that negative charges were spread about a positive charged medium, making atoms neutral. Common Clues: Plum pudding, Electrons had negative charges, disproved by either Rutherford or Mardsen and Geiger The image cannot be displayed. -

Appendix E Nobel Prizes in Nuclear Science
Nuclear Science—A Guide to the Nuclear Science Wall Chart ©2018 Contemporary Physics Education Project (CPEP) Appendix E Nobel Prizes in Nuclear Science Many Nobel Prizes have been awarded for nuclear research and instrumentation. The field has spun off: particle physics, nuclear astrophysics, nuclear power reactors, nuclear medicine, and nuclear weapons. Understanding how the nucleus works and applying that knowledge to technology has been one of the most significant accomplishments of twentieth century scientific research. Each prize was awarded for physics unless otherwise noted. Name(s) Discovery Year Henri Becquerel, Pierre Discovered spontaneous radioactivity 1903 Curie, and Marie Curie Ernest Rutherford Work on the disintegration of the elements and 1908 chemistry of radioactive elements (chem) Marie Curie Discovery of radium and polonium 1911 (chem) Frederick Soddy Work on chemistry of radioactive substances 1921 including the origin and nature of radioactive (chem) isotopes Francis Aston Discovery of isotopes in many non-radioactive 1922 elements, also enunciated the whole-number rule of (chem) atomic masses Charles Wilson Development of the cloud chamber for detecting 1927 charged particles Harold Urey Discovery of heavy hydrogen (deuterium) 1934 (chem) Frederic Joliot and Synthesis of several new radioactive elements 1935 Irene Joliot-Curie (chem) James Chadwick Discovery of the neutron 1935 Carl David Anderson Discovery of the positron 1936 Enrico Fermi New radioactive elements produced by neutron 1938 irradiation Ernest Lawrence -

Henry Ford NERS/BIOE 481 Lecture 01 Introduction
NERS/BIOE 481 Lecture 01 Introduction Michael Flynn, Adjunct Prof Nuclear Engr & Rad. Science Henry Ford Health System [email protected] [email protected] RADIOLOGY RESEARCH I.A – Imaging Systems (6 charts) A) Imaging Systems 1) General Model 2) Medical diagnosis 3) Industrial inspection NERS/BIOE 481 - 2019 2 I.A.1 - General Model – xray imaging Xrays are used to examine the interior content of objects by recording and displaying transmitted radiation from a point source. DETECTION DISPLAY (A) Subject contrast from radiation transmission is (B) recorded by the detector and (C) transformed to display values that are (D) sent to a display device for (E) presentation to the human visual system. NERS/BIOE 481 - 2019 3 I.A.2 - Medical Radiographs Traditional Modern Film-screen Digital Radiograph Radiograph NERS/BIOE 481 - 2019 4 I.A.1 - General Model – radioisotope imaging Radioisotope imaging differs from xray imaging only with repect to the source of radiation and the manner in which radiation reaches the detector DETECTION DISPLAY A B Pharmaceuticals tagged with radioisotopes accumulate in target regions. The detector records the radioactivity distribution by using a multi-hole collimator. NERS/BIOE 481 - 2019 5 I.A.2 - Medical Radisotope image Radioisotope image depicting the perfusion of blood into the lungs. Images are obtained after an intra-venous injection of albumen microspheres labeled with technetium 99m. Anterior Posterior NERS/BIOE 481 - 2019 6 I.A.3 - Industrial Radiography – homeland security Aracor Eagle High energy x-rays and a linear detector are used to scan large vehicles for border inspection NERS/BIOE 481 - 2019 7 I.A.3 - Industrial radiography – battery CT CT image of a lithium battery (Duracell CR2) “Tracking the dynamic morphology of active materials Finegan during operation of et.al., lithium batteries is Advanced essential for Science, identifying causes 2016 (3). -

Historical Background Prepared by Dr, Robin Chaplin Professor of Power Plant Engineering (Retired) University of New Brunswick
1 Historical Background prepared by Dr, Robin Chaplin Professor of Power Plant Engineering (retired) University of New Brunswick Summary: A review of the historical background for the development of nuclear energy is given to set the scene for the discussion of CANDU reactors. Table of Contents 1 Growth of Science and Technology......................................................................................... 2 2 Renowned Scientists............................................................................................................... 4 3 Significant Achievements........................................................................................................ 6 3.1 Niels Bohr........................................................................................................................ 6 3.2 James Chadwick .............................................................................................................. 6 3.3 Enrico Fermi .................................................................................................................... 6 4 Nuclear Fission........................................................................................................................ 7 5 Nuclear Energy........................................................................................................................ 7 6 Acknowledgments................................................................................................................... 8 List of Figures Figure 1 Timeline of significant discoveries -

Absolute Zero, Absolute Temperature. Absolute Zero Is the Lowest
Contents Radioactivity: The First Puzzles................................................ 1 The “Uranic Rays” of Henri Becquerel .......................................... 1 The Discovery ............................................................... 2 Is It Really Phosphorescence? .............................................. 4 What Is the Nature of the Radiation?....................................... 5 A Limited Impact on Scientists and the Public ............................ 6 Why 1896? .................................................................. 7 Was Radioactivity Discovered by Chance? ................................ 7 Polonium and Radium............................................................. 9 Marya Skłodowska .......................................................... 9 Pierre Curie .................................................................. 10 Polonium and Radium: Pierre and Marie Curie Invent Radiochemistry.. 11 Enigmas...................................................................... 14 Emanation from Thorium ......................................................... 17 Ernest Rutherford ........................................................... 17 Rutherford Studies Radioactivity: ˛-and ˇ-Rays.......................... 18 ˇ-Rays Are Electrons ....................................................... 19 Rutherford in Montreal: The Radiation of Thorium, the Exponential Decrease........................................... 19 “Induced” and “Excited” Radioactivity .................................... 20 Elster -

History of Thethermionic Tube / Valve / Vacuum
History of theThermionic Tube / Valve / Vacuum Tube – Page 1 The following notes have been assembled by Phil (VK5SRP) from original material and material from several web sites, including Wikipedia for a class run at the North East Radio Club, South Australia January 2016. In electronics, a vacuum tube, an electron tube, or just a tube (North America), or valve (Britain and some other regions) is a device that controls electric current between electrodes in an evacuated container. Vacuum tubes mostly rely on thermionic emission of electrons from a hot filament or a cathode heated by the filament/heater. This type is called a thermionic tube or thermionic valve. A Photo-tube, however, achieves electron emission through the photoelectric effect. Not all electronic circuit valves/electron tubes are vacuum tubes (evacuated). Gas-filled tubes are similar devices containing a gas, typically at low pressure, which exploit phenomena related to electric discharge in gases, usually without a heater. Although thermionic emission was originally reported in 1873 by Frederick Guthrie, it was Thomas Edison's 1883 investigation that spurred future research, the phenomenon thus becoming known as the "Edison effect". Edison patented what he found, but he did not understand the underlying physics, nor did he have an inkling of the potential value of the discovery. It wasn't until the early 20th century that the rectifying property of such a device was utilised, most notably by John Ambrose Fleming, who used the Diode tube to detect (demodulate) radio signals. Lee De Forest's 1906 "Audion" was also developed as a radio detector, and soon led to the development of the Triode tube. -

Lecture #3, Atomic Structure (Rutherford, Bohr Models)
Welcome to 3.091 Lecture 3 September 14, 2009 Atomic Models: Rutherford & Bohr Periodic Table Quiz 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 Name Grade /10 Image by MIT OpenCourseWare. La Lazy Ce college Pr professors Nd never Pm produce Sm sufficiently Eu educated Gd graduates Tb to Dy dramatically Ho help Er executives Tm trim Yb yearly Lu losses. © source unknown. All rights reserved. This image is excluded from our Creative Commons license. For more information, see http://ocw.mit.edu/fairuse. La Loony Ce chemistry Pr professor Nd needs Pm partner: Sm seeking cannot be referring Eu educated to 3.091! Gd graduate Tb to must be the “other” Dy develop Ho hazardous chemistry professor Er experiments Tm testing Yb young Lu lab assistants. © source unknown. All rights reserved. This image is excluded from our Creative Commons license. For more information, see http://ocw.mit.edu/fairuse. 138.9055 920 57 3455 3 6.146 * 1.10 57 5.577 La [Xe]5d16s2 Lanthanum CEase not I to slave, back breaking to tend; PRideless and bootless stoking hearth and fire. No Dream of mine own precious time to spend Pour'ed More to sate your glutt'nous desire. -
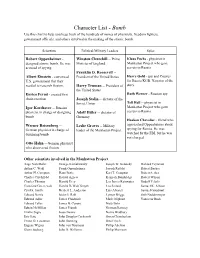
Character List
Character List - Bomb Use this chart to help you keep track of the hundreds of names of physicists, freedom fighters, government officials, and others involved in the making of the atomic bomb. Scientists Political/Military Leaders Spies Robert Oppenheimer - Winston Churchill -- Prime Klaus Fuchs - physicist in designed atomic bomb. He was Minister of England Manhattan Project who gave accused of spying. secrets to Russia Franklin D. Roosevelt -- Albert Einstein - convinced President of the United States Harry Gold - spy and Courier U.S. government that they for Russia KGB. Narrator of the needed to research fission. Harry Truman -- President of story the United States Enrico Fermi - created first Ruth Werner - Russian spy chain reaction Joseph Stalin -- dictator of the Tell Hall -- physicist in Soviet Union Igor Korchatov -- Russian Manhattan Project who gave physicist in charge of designing Adolf Hitler -- dictator of secrets to Russia bomb Germany Haakon Chevalier - friend who Werner Reisenberg -- Leslie Groves -- Military approached Oppenheimer about German physicist in charge of leader of the Manhattan Project spying for Russia. He was designing bomb watched by the FBI, but he was not charged. Otto Hahn -- German physicist who discovered fission Other scientists involved in the Manhattan Project: Aage Niels Bohr George Kistiakowsky Joseph W. Kennedy Richard Feynman Arthur C. Wahl Frank Oppenheimer Joseph Rotblat Robert Bacher Arthur H. Compton Hans Bethe Karl T. Compton Robert Serber Charles Critchfield Harold Agnew Kenneth Bainbridge Robert Wilson Charles Thomas Harold Urey Leo James Rainwater Rudolf Pelerls Crawford Greenewalt Harold DeWolf Smyth Leo Szilard Samuel K. Allison Cyril S. Smith Herbert L. Anderson Luis Alvarez Samuel Goudsmit Edward Norris Isidor I.