Heliothis Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Basis of Pheromonogenesis Regulation in Moths
Chapter 8 Molecular Basis of Pheromonogenesis Regulation in Moths J. Joe Hull and Adrien Fónagy Abstract Sexual communication among the vast majority of moths typically involves the synthesis and release of species-specifc, multicomponent blends of sex pheromones (types of insect semiochemicals) by females. These compounds are then interpreted by conspecifc males as olfactory cues regarding female reproduc- tive readiness and assist in pinpointing the spatial location of emitting females. Studies by multiple groups using different model systems have shown that most sex pheromones are synthesized de novo from acetyl-CoA by functionally specialized cells that comprise the pheromone gland. Although signifcant progress was made in identifying pheromone components and elucidating their biosynthetic pathways, it wasn’t until the advent of modern molecular approaches and the increased avail- ability of genetic resources that a more complete understanding of the molecular basis underlying pheromonogenesis was developed. Pheromonogenesis is regulated by a neuropeptide termed Pheromone Biosynthesis Activating Neuropeptide (PBAN) that acts on a G protein-coupled receptor expressed at the surface of phero- mone gland cells. Activation of the PBAN receptor (PBANR) triggers a signal trans- duction cascade that utilizes an infux of extracellular Ca2+ to drive the concerted action of multiple enzymatic steps (i.e. chain-shortening, desaturation, and fatty acyl reduction) that generate the multicomponent pheromone blends specifc to each species. In this chapter, we provide a brief overview of moth sex pheromones before expanding on the molecular mechanisms regulating pheromonogenesis, and con- clude by highlighting recent developments in the literature that disrupt/exploit this critical pathway. J. J. Hull (*) USDA-ARS, US Arid Land Agricultural Research Center, Maricopa, AZ, USA e-mail: [email protected] A. -

A New Helicoverpa Armigera Nucleopolyhedrovirus Isolate from Heliothis Peltigera (Denis & Schiffermuller) (Lepidoptera: Noctuidae) in Turkey
Turkish Journal of Biology Turk J Biol (2019) 43: 340-348 http://journals.tubitak.gov.tr/biology/ © TÜBİTAK Research Article doi:10.3906/biy-1902-64 A new Helicoverpa armigera Nucleopolyhedrovirus isolate from Heliothis peltigera (Denis & Schiffermuller) (Lepidoptera: Noctuidae) in Turkey Gözde Büşra EROĞLU, Remziye NALÇACIOĞLU, Zihni DEMİRBAĞ* Department of Biology, Faculty of Science, Karadeniz Technical University, Trabzon, Turkey Received: 20.02.2019 Accepted/Published Online: 17.09.2019 Final Version: 14.10.2019 Abstract: This study reports a new Helicoverpa armigera nucleopolyhedrovirus (NPV) isolated from Heliothis peltigera (Denis & Schiffermuller), collected in the vicinity of Adana, Turkey. Infection was confirmed by tissue polymerase chain reaction and sequence analysis. Results showed that dead H. peltigera larvae contain Helicoverpa armigera nucleopolyhedrovirus. Thus, the isolate was named as HearNPV-TR. Microscopy studies indicated that occlusion bodies were 0.73 to 1.66 μm in diameter. The nucleocapsids are approximately 184 × 41 nm in size. The genome of HearNPV-TR was digested with KpnI and XhoI enzymes and calculated as 130.5 kb. Phylogenetic analysis showed that HearNPV-TR has close relation with the H. armigera SNPV-1073 China isolate. The Kimura analysis confirmed that the isolate is a variant of H. armigera NPV. Bioassays were performed using six different concentrations (1 × 310 to 1 × 8 10 occlusion bodies (OBs)/mL)on 2nd instar larvae of H. peltigera, H. armigera, Heliothis viriplaca, Heliothis nubigera. LC50 values were calculated to be 9.5 × 103, 1.9 × 104, 8.6 × 104 and 9.2 × 104 OBs/mL within 14 days, respectively. Results showed that it is a promising biocontrol agent against Heliothinae species. -

SPG2: Biodiversity Conservation (July 2006) 1 1.0 an OVERVIEW
Kent and Medway Structure Plan 2006 mapping out the future Supplementary Planning Guidance SPG2 Biodiversity Conservation July 2006 Strategy and Planning Division/ Environment and Waste Division Environment and Regeneration Directorate Kent County Council Tel: 01622 221609 Email: [email protected] Kent and Medway Structure Plan 2006 Supplementary Planning Guidance (SPG2): Biodiversity Conservation Preface i. The purpose of Supplementary Planning Guidance (SPG) is to supplement the policies and proposals of development plans. It elaborates policies so that they can be better understood and effectively applied. SPG should be clearly cross-referenced to the relevant plan policy or policies which it supplements and should be the subject of consultation during its preparation. In these circumstances SPG may be taken into account as a material consideration in planning decisions. ii. A number of elements of SPG have been produced to supplement certain policies in the Kent and Medway Structure Plan. This SPG supplements the following policies: • Policy EN6: International and National Wildlife Designations • Policy EN7: County and Local Wildlife Designations • Policy EN8: Protecting, Conserving and Enhancing Biodiversity • Policy EN9: Trees, Woodland and Hedgerows iii. This SPG has been prepared by Kent County Council working in partnership with a range of stakeholders drawn from Kent local authorities and other relevant agencies. iv. A draft of this SPG was subject to public consultation alongside public consultation on the deposit draft of the Kent and Medway Structure Plan in late 2003. It has been subsequently revised and updated prior to its adoption. A separate report provides a statement of the consultation undertaken, the representations received and the response to these representations. -

A New Larval Parasitoid of Heliothis Peltigera (Denis & Schiffermüller)
Türk. Biyo. Mücadele Derg. 2020, 11(1):83-89 DOI: 10.31019/tbmd.628853 ISSN 2146-0035-E-ISSN 2548-1002 Orijinal araştırma (Original article) A new larval parasitoid of Heliothis peltigera (Denis & Schiffermüller) (Lepidoptera: Noctuidae), Aleiodes (Chelonorhogas) miniatus (Herrich-Schäffer) (Hymenoptera: Braconidae: Rogadinae) Sevgi AYTEN1*, Ahmet BEYARSLAN2, Selma ÜLGENTÜRK3 Heliothis peltigera (Denis & Schiffermüller) (Lepidoptera: Noctuidae)’nın yeni bir larva parazitoiti; Aleiodes (Chelonorhogas) miniatus (Herrich-Schäffer) (Hymenoptera: Braconidae: Rogadinae) Özet: Aspir, geniş kullanım alanlarına sahip endüstriyel bir bitkidir. Heliothis peltigera (Denis & Schiffermüller, 1775) (Lepidoptera: Noctuidae) Ankara ilinde önemli bir aspir zararlısıdır. Bu türün larvalarının Aleiodes (Chelonorhogas) miniatus (Herrich-Schäffer, 1838) (Hymenoptera: Braconidae: Rogadinae) tarafından parazitlendiği tespit edilmiştir. Bu parazitoit türünün konukçusu Dünya’ da ilk defa ortaya konmuştur. Anahtar kelimeler: Heliothis peltigera, Aleiodes miniatus, Carthamus tinctorius, aspir, ilk kayıt Abstract: Safflower is a plant grown for a wide range of industrial uses. A survey revealed that the larvae of Heliothis peltigera (Denis & Schiffermüller, 1775) (Lepidoptera: Noctuidae), an important safflower pest in Ankara Province, Turkey, are parasitized by Aleiodes (Chelonorhogas) miniatus (Herrich-Schäffer, 1838) (Hymenoptera: Braconidae: Rogadinae). This is the first report of this host-parasitoid relationship worldwide. Keywords: Heliothis peltigera, Aleiodes -

Climate-Smart Agriculture Training Manual for Agricultural Extension Agents in Kenya
In partnership with Ministry of Agriculture, Livestock and Fisheries Climate-Smart Agriculture Training Manual for Agricultural Extension Agents in Kenya Climate-Smart Agriculture | Training Manual for Agricultural Extension Agents in Kenya 1 Climate-Smart Agriculture | Training Manual for Agricultural Extension Agents in Kenya 2 CLIMATE-SMART AGRICULTURE TRAINING MANUAL FOR AGRICULTURAL EXTENSION AGENTS IN KENYA Author: BarrackO . Okoba MINISTRY OF AGRICULTURE LIVESTOCK AND FISHERIES FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS KENYA, 2018 Climate-Smart Agriculture | Training Manual for Agricultural Extension Agents in Kenya 3i Required Citation: FAO, Ministry of Agriculture, Livestock and Fisheries, 2018. Climate Smart Agriculture - Train- ing Manual for Extension Agents in Kenya. ISBN 978-92-5-130780-9 © FAO, 2018 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The designations employed and the presentation of material does not imply the expression of any opinion whatsoever on the part of FAO concerning the legal or constitutional status of any country, territory or sea area concerning the delimitation of frontiers,. FAO encourages the use, reproduction and dissemination of material in this information product. -
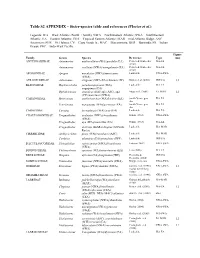
Table S2 APPENDIX – Sister-Species Table and References (Floeter Et Al.)
Table S2 APPENDIX – Sister-species table and references (Floeter et al.) Legends: WA = West Atlantic (North + South); NWA = Northwestern Atlantic; SWA = Southwestern Atlantic; EA = Eastern Atlantic; TEA = Tropical Eastern Atlantic; MAR = mid-Atlantic Ridge; ASC = Ascension; SHE = St. Helena; CV = Cape Verde Is.; MAC = Macaronesia; BER = Bermuda; IO = Indian Ocean; IWP = Indo-West Pacific. Figure Family Genus Species Reference Type (ms) ANTENNARIIDAE Antennarius multiocellatus (WA)/pardalis (EA) Pietsch & Grobecker WA-EA (1987) Antennarius ocellatus (NWA)/senegalensis (EA) Pietsch & Grobecker WA-EA (1987) APOGONIDAE Apogon maculatus (NWA)/americanus Look alike NWA-SWA (SWA) AULOSTOMIDAE Aulostomus strigosus (SWA-EA)/chinensis (IP) Bowen et al. (2001) IWP-EA 12 BLENNIIDAE Hypleurochilus pseudoaequipinnis (WA)/ Look alike WA-EA aequipinnis (EA) Ophioblennius atlanticus (EA)/ sp1 (ASC), sp2 Muss et al. (2001) EA-MAR 12 (CV)/macclurei (NWA) CARANGIDAE Hemicaranx amblyrhynchus (WA)/bicolor (EA) Smith-Vaniz, pers WA-EA obs. Trachinotus marginatus (WA)/goreensis (EA) Smith-Vaniz, pers WA-EA obs. CARAPIDAE Carapus bermudensis (WA)/acus (EA) Look alike WA-EA CHAETODONTIDAE Prognathodes aculeatus (NWA)/brasiliensis Hubbs (1963) NWA-SWA (SWA) Prognathodes aya (WA)/marcellae (EA) Hubbs (1963) WA-EA Prognathodes dichrous (MAR)/obliquus (St Paul's Look alike WA-MAR Rocks) CIRRHITIDAE Amblycirrhitus pinos (WA)/earnshawi (ASC) Look alike WA-MAR Cirrhitus atlanticus (EA)/pinnulatus (IWP) Look alike IWP-EA DACTYLOSCOPIDAE Platygillellus rubrocinctus (NWA)/brasiliensis Feitoza (2002) NWA-SWA (SWA) DIODONTIDAE Chilomycterus spinosus (WA)/mauretanicus (EA) Leis (2006) WA-EA DREPANIDAE Drepane africana (EA)/longimana (IWP) Heemstra & IWP-EA Heemstra (2004) GOBIESOCIDAE Tomicodon fasciatus (NWA)/australis (SWA) Briggs, pers com NWA-SWA GOBIIDAE Elacatinus figaro (SWA)/randalli (NWA) Sazima et al. -

Pulse Crops of the World and Their Important Insect Pests.
u* ,'Eti:ati brary TJTU OF THESIS/TITRE DE LA TH& "Pulse Crops of the World and their Important Insect pestsw UN~VERS~~/~N~VERSIT~ Simon Fraser University 1 DEGREE FOR WHICH THESIS WAS SENTEW. cnmEpour Mom mm $5 wTPR~SENT~E . *aster of pest ~mag-nt NAME OF WlSOR/NOW DU DIRECTEUR DE THiSE J-M* Permission is hereby grated to the NATI~ALLIBRARY OF Llutwisdtion m,b.r I# prdssnte, wcordde b I~@BUOTH&- CANADA to microfilm this thesis and to lend or sell mpin QUE NATIONALF DU C.)NADA ds mi0r dketMss et C of the film. * de prbter w do v'sndio dss sxemplsirrs du film The author lsrsus aha publication rights, ad neither the . f'a& re r4s.m /eg 4utms d. p(rblic8tion: nl h wise mpr&ced without the wthor's mitten permissiar. IMPORTANT INSECT PESTS Carl Edmond Japlin R B.A., Antioch College, 1973 - A PROJECT SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF PEST MANAGEHENT in the Department , of' . - 4 F+ , @ Carl Edmond Joplin Simon F~aaerUniversity -8 -d. - - - - - -- - -- .-- -, 197'4 = _ s -- -- -- -- -- -- -- --- - -- % All ,rights reserved. This the848 'may not be reproduced in-whole or in part by photocopy or other'means, without permission of the author. APPROVAL . Name: -Carl Edmond Joplin L Degree: Master of Pest Management Title of Project: Pulse Crops of the World and their Important Insect Pests Examining Committee: , r*. Chairman: John S. Barlow L -- Johii M. Webster Senior Supervisor Thelma 'Finlayson dames E. She * Hubert R. Kwarthy Head, Entomology Section Vancouver gesearch sf at ion Agriculture Canada Date Approved : ! /?74 c . -

The Anti-Lebanon Ridge As the Edge of the Distribution Range for Euro
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Kravchenko, V. D.; Friedman, A.-L.-L.; Müller, G. C. The Anti-Lebanon ridge as the edge of the distribution range for Euro-Siberian and Irano- Turanian faunistic elements in the Mediterranean biome: A case study (Lepidoptera: Noctuidae) SHILAP Revista de Lepidopterología, vol. 45, núm. 180, diciembre, 2017, pp. 639-650 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45553890016 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative SHILAP Revta. lepid., 45 (180) diciembre 2017: 639-650 eISSN: 2340-4078 ISSN: 0300-5267 The Anti-Lebanon ridge as the edge of the distribution range for Euro-Siberian and Irano-Turanian faunistic elements in the Mediterranean biome: A case study (Lepidoptera: Noctuidae) V. D. Kravchenko, A.-L.-L. Friedman & G. C. Müller Abstract The Lebanon and Anti-Lebanon ridges are located in the middle of a narrow “Mediterranean ecozone” corridor stretching along the Levantine coast. Both ridges are high enough to feature a complete range of altitude zones, which includes an alpine tragacanth belt (> 2000 m a.s.l.). The southernmost part of the Anti-Lebanon ridge is situated in the northernmost part of Israel. Among the 548 Israeli Noctuidae species, 106 species (21%) occur only in this small mountainous area. Among them, 17 are endemic and the populations of the remaining 89 species are at the edge of their distribution range. -

Neuroscience Needs Ethology: the Marked Example of the Moth Pheromone System
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 16 July 2020 doi:10.20944/preprints202007.0357.v1 Review Neuroscience needs ethology: the marked example of the moth pheromone system Hervé Thevenon 1 1 Affiliation: Spascia; [email protected] 2 Correspondence: [email protected] Abstract: The key premise of translational studies is that knowledge gained in one animal species can be transposed to other animals. So far translational bridges have mainly relied on genetic and physiological similarities, in experimental setups where behaviours and environment are often oversimplified. These simplifications were recently criticised for decreasing the intrinsic value of the published results. The inclusion of wild behaviour and rich environments in neuroscience experimental designs is difficult to achieve because no animal model has it all. As an example, the genetic toolkit of moths species is virtually non-existent when compared to C. elegans, rats, mice, or zebrafish, however the balance is reversed for wild behaviours. The ethological knowledge gathered about the moth was instrumental for designing natural-like auditory stimuli, that were used in association with electrophysiology in order to understand how moths use these variable sounds produced by their predators in order to trump death. Conversely, we are still stuck with understanding how male moths make sense of their complex and diffuse olfactory landscape in order to locate conspecific females up to several hundred meters away, and precisely identify a conspecific in a sympatric swarm in order to reproduce. This systemic review articulates the ethological knowledge pertaining to this unresolved problem and leverages the paradigm to gain insight into how male moths process sparse and uncertain environmental sensory information. -

D--Accb-IJPP1-IJPP 5 (2)
Click www.researchjournal.co.in/online/subdetail.html to purchase. INTERNATIONAL JOURNAL OF PLANT PROTECTION | VOLUME 5 | ISSUE 2 | OCTOBER, 2012 | 312-314 IJPP RESEARCH ARTICLE Survival of Colletotrichum truncatum in seeds and crop debris of greengram [Vigna radiata (L.) Wilczek] SUNIL KULKARNI1* AND V. I. BENAGI2 1*Department of Plant Pathology, Krishi Vigyan Kendra, [U.A.S.(R.)], BIDAR (KARNATAKA) INDIA 2Department of Plant Pathology, University of Agricultural Sciences, DHARWAD (KARNATAKA) INDIA ARITCLE INFO ABSTRACT Received : 04.05.2012 The per cent viability of conidia of Colletotrichum truncatum in crop debris was significantly Revised : 31.05.2015 affected by duration of storage as well as different storage conditions. The conidia survived for Accepted : 27.08.2012 a maximum of 360 days under freeze (4 - 50C) conditions and least survivability of 90 days Key Words : under field condition (28 - 300C). In case of infected seeds, the pathogen survived up to the next Greengram, crop season (12 months) but the survivability decreased with lapse of time. However, the Survival, Anthracnose, germination percentage of the seeds increased with storage time. Colletotrichum truncatum, Crop debris How to view point the article : Kulkarni, Sunil and Benagi, V.I. (2012). Survival of Colletotrichum truncatum in seeds and crop debris of greengram [Vigna radiata(L.) Wilczek]. Internat. J. Plant *Corresponding author: Protec., 5(2) : 312-314. [email protected] INTRODUCTION post crop season to observe the role of infected seeds and crop debris in the perpetuation of the pathogen from season Greengram [Vigna radiata (L.) Wilczek] is one of the to season. important pulse crops of India and is cultivated in 32.99 lakh ha with production of 13.74 lakh tonnes with a productivity of 417 kg per ha, while in Karnataka, the area under greengram MATERIALS AND METHODS was 1.77 lakh ha and production of 0.71 lakh tonnes and The present investigation on viability and survival of C. -
![Lablab Purpureus (L.) Sweet] B.S](https://docslib.b-cdn.net/cover/5242/lablab-purpureus-l-sweet-b-s-1325242.webp)
Lablab Purpureus (L.) Sweet] B.S
Research Paper : Pests and predators activity on new variety of dolichos bean [Lablab purpureus (L.) Sweet] B.S. RAJENDRA PRASAD, M. BYRE GOWDA , C.S. JAGADEESH BABU, G. N. VEERA KUMAR AND C.K. PRAMILA International Journal of Plant Protection, Vol. 4 No. 2 (October, 2011) : 385-389 See end of the article for authors’ affiliations SUMMARY Correspondence to : A study was carried out to investigate the incidence of different insect pests and predators on new B.S. RAJENDRA variety, HA -4 of dolichos bean. The results of the field study revealed that 15 insect pests belonging to PRASAD nine different families of five orders and five species of predators belonging to five different families AICRP (Pigeonpea) coming under four orders. The sucking pest population was found throughout the year. The peak University of Agricultural Sciences, population of aphids (49.00/3 leaves), pentatomid (5.20/5 plants) and coreid bugs (11.20/5 plants) were G.K.V.K., observed on 60 days after sowing (DAS). Whereas, eurybrachid bugs (5.20/5 plants) recorded at 50 BENGALURU DAS. Among the pod borer complex, higher pod damage due to Helicoverpa armigera , Maruca vitrata (KARNATAKA) and Lampides boeticus was 20.43, 16.66 and 10.20 per cent pod damage, respectively on 80 DAS, INDIA whereas, Callosobruchus theobromae (12.55 %) was observed on 90 DAS. The important predator’s Email : rajendrayadav viz., robber fly, coccinellids, syrphids, green lacewing and dragonfly were prominent ones. The activity [email protected] of predators was high between 40 and 60 DAS and population decline was observed thereafter. -

Reef Fisheries and Underwater Surveys Indicate Overfishing of a Brazilian Coastal Island
Research Letters Natureza & Conservação 8(2):151-159, December 2010 Copyright© 2010 ABECO Handling Editor: Sergio R. Floeter Brazilian Journal of Nature Conservation doi: 10.4322/natcon.00802008 Reef Fisheries and Underwater Surveys Indicate Overfishing of a Brazilian Coastal Island Hudson Tercio Pinheiro*, Jean-Christophe Joyeux & Agnaldo Silva Martins Departamento de Oceanografia e Ecologia, Universidade Federal do Espírito Santo, Vitória, ES, Brasil Abstract The preoccupation about fishing effects on marine ecosystems has increased sharply over the last three decades. However, little is known about the impact of multi-gear artisanal and recreational fisheries on the structure of local reef fish communities in Brazil. Fishing activities around a Brazilian coastal island were monitored while reef fish density was censused during underwater surveys (UVC). The links between frequency of capture, intensity at which species are wished and UVC density were explored. Species were classified according to their frequency of capture as regular, occasional and rare, and classified according to the intensity at which they are wished (based on size and price), as highly targeted, average and non-targeted species. Ninety-seven species were caught by fishing, the majority of them being either rarely caught or non-targeted. Nineteen species were highly targeted but rarely caught. The highly targeted species showed extremely low density in the UVC. These results put in question the sustainability of the local fishing activities. The predominance of non-targeted species in the catches and in the reefs environment studied supports the expectation that these species will be more and more captured, thus collaborating to further change the structure of the reef community.