Silver Perch
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2016-2017 Native Fish Stocking Plan for Dams and Lakes
2016/2017 NATIVE FISH STOCKING PLAN FOR DAMS AND LAKES There are many impoundments and reservoirs suitable for native fish stocking throughout NSW and over the last two decades a large number of excellent recreational fisheries have been established. To ensure that the best use continues to be made of publicly funded fish stocking programs, Department of Primary Industries (DPI) is seeking input from people who have an interest in the State’s stocked native freshwater fisheries. The attached draft native fish stocking plan has been prepared for consideration by the recreational fishing community. Fish are stocked from Government hatcheries as a service to the anglers of NSW. Locations are selected based on recent stocking history and experience with those waters. The plan is also developed in accordance with the policies and guidelines set out in the Environmental Impact Statement and Fishery Management Strategy (FMS) on freshwater fish stocking in NSW. The water quality and storage status of impoundments will also be assessed prior to stocking and where necessary changes will be made. Please note: Planned fish release figures listed in the attached tables are targets only, and may be exceeded, or not attained, depending on hatchery production. Other seasonal factors such as water quality issues or unforeseen circumstances could preclude planned fish releases. As a result, allocations may be amended prior to release. Impoundments are listed as Priority 1 or 2. Priority 1 impoundments support large recreational fisheries or have not received stockings in recent years. Priority 2 impoundments are either smaller fisheries, suffer intermittent water quality issues or have recently received large stockings of that species. -

Namoi River Salinity
Instream salinity models of NSW tributaries in the Murray-Darling Basin Volume 3 – Namoi River Salinity Integrated Quantity and Quality Model Publisher NSW Department of Water and Energy Level 17, 227 Elizabeth Street GPO Box 3889 Sydney NSW 2001 T 02 8281 7777 F 02 8281 7799 [email protected] www.dwe.nsw.gov.au Instream salinity models of NSW tributaries in the Murray-Darling Basin Volume 3 – Namoi River Salinity Integrated Quantity and Quality Model April 2008 ISBN (volume 2) 978 0 7347 5990 0 ISBN (set) 978 0 7347 5991 7 Volumes in this set: In-stream Salinity Models of NSW Tributaries in the Murray Darling Basin Volume 1 – Border Rivers Salinity Integrated Quantity and Quality Model Volume 2 – Gwydir River Salinity Integrated Quantity and Quality Model Volume 3 – Namoi River Salinity Integrated Quantity and Quality Model Volume 4 – Macquarie River Salinity Integrated Quantity and Quality Model Volume 5 – Lachlan River Salinity Integrated Quantity and Quality Model Volume 6 – Murrumbidgee River Salinity Integrated Quantity and Quality Model Volume 7 – Barwon-Darling River System Salinity Integrated Quantity and Quality Model Acknowledgements Technical work and reporting by Perlita Arranz, Richard Beecham, and Chris Ribbons. This publication may be cited as: Department of Water and Energy, 2008. Instream salinity models of NSW tributaries in the Murray-Darling Basin: Volume 3 – Namoi River Salinity Integrated Quantity and Quality Model, NSW Government. © State of New South Wales through the Department of Water and Energy, 2008 This work may be freely reproduced and distributed for most purposes, however some restrictions apply. Contact the Department of Water and Energy for copyright information. -

Sunwater Irrigation Price Review: 2012-17 Volume 2 Macintyre Brook Water Supply Scheme
Final Report SunWater Irrigation Price Review: 2012-17 Volume 2 Macintyre Brook Water Supply Scheme April 2012 Level 19, 12 Creek Street Brisbane Queensland 4000 GPO Box 2257 Brisbane Qld 4001 Telephone (07) 3222 0555 Facsimile (07) 3222 0599 [email protected] www.qca.org.au © Queensland Competition Authority 2012 The Queensland Competition Authority supports and encourages the dissemination and exchange of information. However, copyright protects this document. The Queensland Competition Authority has no objection to this material being reproduced, made available online or electronically but only if it is recognised as the owner of the copyright and this material remains unaltered. Queensland Competition Authority Table of Contents TABLE OF CONTENTS PAGE GLOSSARY III EXECUTIVE SUMMARY IV 1. MACINTYRE BROOK WATER SUPPLY SCHEME 1 1.1 Scheme Description 1 1.2 Bulk Water Infrastructure 1 1.3 Network Service Plans 2 1.4 Consultation 2 2. REGULATORY FRAMEWORK 4 2.1 Introduction 4 2.2 Draft Report 4 2.3 Submissions Received from Stakeholders on the Draft Report 6 2.4 Authority’s Response to Submissions Received on the Draft Report 6 3. PRICING FRAMEWORK 7 3.1 Tariff Structure 7 3.2 Water Use Forecasts 9 3.3 Tariff Groups 10 3.4 Continuous Sharing Arrangements 11 4. RENEWALS ANNUITY 12 4.1 Background 12 4.2 SunWater’s Opening ARR Balance (1 July 2006) 13 4.3 Past Renewals Expenditure 14 4.4 Opening ARR Balance (at 1 July 2012) 26 4.5 Future Renewals Expenditure 27 4.6 SunWater’s Consultation with Customers 39 4.7 Allocation of Renewals Expenditure According to WAE Priority 40 4.8 Calculating the Renewals Annuity 44 5. -

New South Wales Class 1 Load Carrying Vehicle Operator’S Guide
New South Wales Class 1 Load Carrying Vehicle Operator’s Guide Important: This Operator’s Guide is for three Notices separated by Part A, Part B and Part C. Please read sections carefully as separate conditions may apply. For enquiries about roads and restrictions listed in this document please contact Transport for NSW Road Access unit: [email protected] 27 October 2020 New South Wales Class 1 Load Carrying Vehicle Operator’s Guide Contents Purpose ................................................................................................................................................................... 4 Definitions ............................................................................................................................................................... 4 NSW Travel Zones .................................................................................................................................................... 5 Part A – NSW Class 1 Load Carrying Vehicles Notice ................................................................................................ 9 About the Notice ..................................................................................................................................................... 9 1: Travel Conditions ................................................................................................................................................. 9 1.1 Pilot and Escort Requirements .......................................................................................................................... -

Pdf\Baffle Boyne Calliope Kolan.Pdf) N
!! Rockhampton 0 5 10 20 30 40 50 Legend !! BROADMEADOWS Keppel !! Automatic rainfall station (RN) Kilometres F !! Manual/Daily rainfall station (DN) itz Bay r Map projection: Geographical Lat and Lon (GDA94). o Automatic river height station (RV) y *# Cape R Capricorn # Manual river height station (RV) Midgee ## !! +! Forecast site (quantitative) Curtis Forecast site (qualitative) PORT ALMA +! #TIDE TM !R !. !( Ck Population centre (large, medium, small) !! erma at Ink n Ck Bo River, creek BAJOOL !! k !POST OFFICE Coral Sea Major highway C Island !( Main road BR Marmor UCE Basin boundary C !! u Catchment boundary SOUTH !! DARTS CK r H ti n W s a Y Dam, lake, water body l g a R MOUNT Swamp !!( !! RAGLAN CK LARCOM! Mt Larcom !! AUCKLAND POINT TM# Gladstone !( TIDE TM # Note: Base spatial data shown in this map is obtained from Yarwun !R!! GLADSTONE PACIFIC Geoscience Australia and Queensland Department of Natural GLADSTONE!! #TIDE TM (AWS) Resources, Mines and Energy. G # GLADSTONE RADAR C L r h a a AWS/AL a !( r v POLICE CK Port n Bracewell c e o l n m AL Curtis el Basin Locality C C k k !! ! #BOYNE ISLAND AL Townsville Qld border, R CASTLEHOPE coastline A !! OCEAN ! l Bowen m #TM Basin HAZELDEAN !! Rodds Bay a #!( boundary CALLIOPE CALLIOPE Calliope !! ! ! FIG pe ! !! !! C io STATION BENARABY AL Mackay TREE l (STOWE RD) AL # k l a C k C !! AWOONGA DAM k # C e AL/HW TM WY l b ! H Awoonga ! ! u I Emerald Rockhampton o Dam ve UPPER BELL CK D r ag !! IVERAGH BOROREN-IVERAGH Bustard AL s h AL !! N m RAIL TM Bay o !! SEVENTEEN SEVENTY ! O T ! Bundaberg S k # !! SPRINGS C B !( W Seventeen Seventy R A MARLUA AL !! U D ! ! k # ! MT MONGREL C C k !! FERNDALE ! Gympie UPPER RAINBOW ! E Charleville ! !! C AL E Roma AL B st CA er o . -

Dubbo Zirconia Project
Dubbo Zirconia Project Aquatic Ecology Assessment Prepared by Alison Hunt & Associates September 2013 Specialist Consultant Studies Compendium Volume 2, Part 7 This page has intentionally been left blank Aquatic Ecology Assessment Prepared for: R.W. Corkery & Co. Pty Limited 62 Hill Street ORANGE NSW 2800 Tel: (02) 6362 5411 Fax: (02) 6361 3622 Email: [email protected] On behalf of: Australian Zirconia Ltd 65 Burswood Road BURSWOOD WA 6100 Tel: (08) 9227 5677 Fax: (08) 9227 8178 Email: [email protected] Prepared by: Alison Hunt & Associates 8 Duncan Street ARNCLIFFE NSW 2205 Tel: (02) 9599 0402 Email: [email protected] September 2013 Alison Hunt & Associates SPECIALIST CONSULTANT STUDIES AUSTRALIAN ZIRCONIA LTD Part 7: Aquatic Ecology Assessment Dubbo Zirconia Project Report No. 545/05 This Copyright is included for the protection of this document COPYRIGHT © Alison Hunt & Associates, 2013 and © Australian Zirconia Ltd, 2013 All intellectual property and copyright reserved. Apart from any fair dealing for the purpose of private study, research, criticism or review, as permitted under the Copyright Act, 1968, no part of this report may be reproduced, transmitted, stored in a retrieval system or adapted in any form or by any means (electronic, mechanical, photocopying, recording or otherwise) without written permission. Enquiries should be addressed to Alison Hunt & Associates. Alison Hunt & Associates RW CORKERY & CO. PTY. LIMITED AUSTRALIAN ZIRCONIA LTD Dubbo Zirconia Project Aquatic Ecology Final September 2013 SPECIALIST CONSULTANT STUDIES AUSTRALIAN ZIRCONIA LTD Part 7: Aquatic Ecology Assessment Dubbo Zirconia Project Report No. 545/05 SUMMARY Alison Hunt & Associates Pty Ltd was commissioned by RW Corkery & Co Pty Limited, on behalf of Australian Zirconia Limited (AZL), to undertake an assessment of aquatic ecology for the proposed development of the Dubbo Zirconia Project (DZP), which would be located at Toongi, approximately 25 km south of Dubbo in Central West NSW. -

Ewater SOFTWARE CONFERENCE 2017 MODELLING ENVIRONMENTAL WATER DEMAND in Ewater SOURCE
eWATER SOFTWARE CONFERENCE 2017 MODELLING ENVIRONMENTAL WATER DEMAND IN eWATER SOURCE Authors: Mahmudul Haque, Golam Kibria & Mahes Maheswaran Presented by: Dr Mahmudul Haque, CPEng, NER Hydrologist, WaterNSW WaterNSW: We are Australia's largest water supplier We own and operate 42 large dams, pipelines and the state’s rivers We supply water to regional towns, irrigators, Sydney Water Corporation and local water utilities We develop infrastructure solutions for water supply security and reliability Website: http://www.waternsw.com.au/home 2 Outline: Macquarie Valley Environmental flow requirements Environmental flow rules Modelling in eWater Source Results 3 Macquarie Valley: Burrendong = Koala 74000 km2 4 ENVIRONMENTAL FLOW REQUIREMENTS Environmental Water/Flow (in a year): Two distinct types (Burrendong Dam): 1. Environmental Water Allowance (EWA)/Plan Water Total: 160 GL/year (not license, has GS characteristics) - 96 GL as translucent flow -If GS allocation is 28%, - 64 GL as active flow then EWA has same 28% allocation 2. Adaptive Environmental Water(AEW)/Held Environmental-It can also carryoverWater(HEW) water Total: 174.5 GL/year up to 100% as like GS - 48.5 GL as NSW purchase GS license - 126 GL Commonwealth Govt. purchase GS license 6 Environmental Water/Flow (in a year): Burrendong dam: (i) The Macquarie EFRG sought and received approval for changes to the apportionment between Active and Translucent components from 40%/60% to 60%/40% in the mid-2000s. The releases from the Active component and any other held environmental water are typically made as recommended by the EFRG . (ii) Releases from the Translucent sub-allowance are generally consistent with WSP triggers. -

Fisheries Management (Authority to Fish Silver Perch) Order 2007
Fisheries Management (Authority to Fish Silver Perch) Order 2007 As at 18 January 2008 I, the Minister for Primary Industries, make the following Order under section 221IA of the Fisheries Management Act 1994. Dated, this 17th day of December 2007. Minister for Primary Industries Explanatory note The silver perch is listed as a vulnerable species (which is a category of threatened species) under the Fisheries Management Act 1994. The Act enables the Minister for Primary Industries to make an order authorising a class of persons to carry out an activity that may result in harm to a threatened species, population or ecological community or damage to its habitat, subject to the Minister's compliance with the requirements of Subdivision 1A of Division 6 of Part 7A of the Act. The object of this Order is to authorise recreational fishers to fish for silver perch in specified waters, subject to compliance with any applicable fishing regulatory controls. This Order is made under section 221IA of the Fisheries Management Act 1994. 1 Name of Order This Order is the Fisheries Management (Authority to Fish Silver Perch) Order 2007. 2 Commencement This Order takes effect on the day that it is published in the Gazette. 3 Activities authorised by this Order (1) Recreational fishers may take silver perch, or possess silver perch taken, from the following bodies of water, or carry out any routine activity in that connection, subject to compliance with any applicable fishing regulatory controls: Ben Chifley Dam Jounama Pondage Blowering Dam Keepit Dam Burrendong Dam Lake Albert Burrinjuck Dam Lake Wyangan Chaffey Dam Pindari Dam Copeton Dam Split Rock Dam Glenbawn Dam Windamere Dam Glennies Creek Wyangla Dam Dam Googong Dam Yass Weir (2) In this clause, "fishing regulatory controls" has the same meaning as in Division 5 of Part 5 of the Environmental Planning and Assessment Act 1979. -
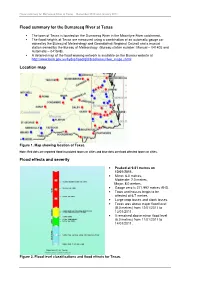
Flood Summary for the Dumaresq River at Texas Location Map Flood
Flood summary for Dumaresq River at Texas – December 2010 and January 2011 Flood summary for the Dumaresq River at Texas • The town of Texas is located on the Dumaresq River in the Macintyre River catchment. • The flood heights at Texas are measured using a combination of an automatic gauge co- owned by the Bureau of Meteorology and Goondiwindi Regional Council and a manual station owned by the Bureau of Meteorology. (Bureau station number: Manual – 041403 and Automatic – 041548). • A detailed map of the flood warning network is available on the Bureau website at http://www.bom.gov.au/hydro/flood/qld/brochures/river_maps.shtml Location map Figure 1. Map showing location of Texas. Note: Red dots are reported flood inundated towns or cities and blue dots are flood affected towns or cities. Flood effects and severity • Peaked at 9.21 metres on 12/01/2011. • Minor: 6.0 metres, Moderate: 7.0 metres, Major: 8.0 metres. • Gauge zero is 271.997 metres AHD. • Town and houses begin to be affected at 6.7 metres. • Large crop losses and stock losses. • Texas was above major flood level (8.0 metres) from 12/01/2011 to 13/01/2011. • It remained above minor flood level (6.0 metres) from 11/01/2011 to 14/01/2011. Figure 2. Flood level classifications and flood effects for Texas. Flood summary for Dumaresq River at Texas – December 2010 and January 2011 Rainfall summary • In excess of 400mm of rainfall was recorded in the upper reaches of the Macintyre River catchment during December 2010 and January 2011. -

Inglewood Shire Handbook
INGLEWOOD SHIRE HANDBOOK An Inventory of the Agricultural Resources and Production of Inglewood Shire, Queensland Queensland Department of Primary Industries November 1977 Queensland Government Technical Report This report is a scanned copy and some detail may be illegible or lost. Before acting on any information, readers are strongly advised to ensure that numerals, percentages and details are correct. This report is intended to provide information only on the subject under review. There are limitations inherent in land resource studies, such as accuracy in relation to map scale and assumptions regarding socio-economic factors for land evaluation. Before acting on the information conveyed in this report, readers should ensure that they have received adequate professional information and advice specific to their enquiry. While all care has been taken in the preparation of this report neither the Queensland Government nor its officers or staff accepts any responsibility for any loss or damage that may result from any inaccuracy or omission in the information contained herein. © State of Queensland 1977 For information about this report contact [email protected] INGLEWOOD SHIRE HANDBOOK An Inventory of the Agricultural Resources and Production of Ingle wood Shire, Queensland Compiled by: G. H. Malcolmson, District Adviser, Inglewood. Edited by: P. L. Lloyd, Extenson Officer, Brisbane. Published by: Queensland Department of Primary Industries. November 1977 FOREWORD The Shire Handbook was conceived in the mid-1960s. A limited number of a series was printed for use by officers of the Department of Primary Industries to assist them in their planning of research and extension programmes. The Handbooks created wide interest and, in response to public demand, it was decided to publish progressively a new updated series. -

The Murray–Darling Basin Basin Animals and Habitat the Basin Supports a Diverse Range of Plants and the Murray–Darling Basin Is Australia’S Largest Animals
The Murray–Darling Basin Basin animals and habitat The Basin supports a diverse range of plants and The Murray–Darling Basin is Australia’s largest animals. Over 350 species of birds (35 endangered), and most diverse river system — a place of great 100 species of lizards, 53 frogs and 46 snakes national significance with many important social, have been recorded — many of them found only in economic and environmental values. Australia. The Basin dominates the landscape of eastern At least 34 bird species depend upon wetlands in 1. 2. 6. Australia, covering over one million square the Basin for breeding. The Macquarie Marshes and kilometres — about 14% of the country — Hume Dam at 7% capacity in 2007 (left) and 100% capactiy in 2011 (right) Narran Lakes are vital habitats for colonial nesting including parts of New South Wales, Victoria, waterbirds (including straw-necked ibis, herons, Queensland and South Australia, and all of the cormorants and spoonbills). Sites such as these Australian Capital Territory. Australia’s three A highly variable river system regularly support more than 20,000 waterbirds and, longest rivers — the Darling, the Murray and the when in flood, over 500,000 birds have been seen. Australia is the driest inhabited continent on earth, Murrumbidgee — run through the Basin. Fifteen species of frogs also occur in the Macquarie and despite having one of the world’s largest Marshes, including the striped and ornate burrowing The Basin is best known as ‘Australia’s food catchments, river flows in the Murray–Darling Basin frogs, the waterholding frog and crucifix toad. bowl’, producing around one-third of the are among the lowest in the world. -

Rural Irrigation Price Review 2020–24 Part A: Overview
Final report Rural irrigation price review 2020–24 Part A: Overview January 2020 © Queensland Competition Authority 2020 The Queensland Competition Authority supports and encourages the dissemination and exchange of information. However, copyright protects this document. The Queensland Competition Authority has no objection to this material being reproduced, made available online or electronically but only if it is recognised as the owner of the copyright2 and this material remains unaltered. Queensland Competition Authority Contents Contents EXECUTIVE SUMMARY III Scope of our review iii Approach iii Prices iii Revenue and cost risks v Approach to apportioning dam safety upgrade capex v Costs vi Recommendations vi 1 OVERVIEW OF OUR APPROACH 1 1.1 Background 1 1.2 Referral 1 1.3 Irrigation services 2 1.4 Key regulatory obligations 3 1.5 Our approach to the investigation and recommending prices 3 1.6 Review process 5 2 PRICING FRAMEWORK 6 2.1 Introduction 6 2.2 Scope of our investigation 6 2.3 Matters we are required to consider in undertaking our investigation 9 2.4 Approach 10 2.5 Stakeholders' submissions 10 2.6 Relevant matters for this investigation 11 2.7 Approach to bill moderation and the transition to lower bound prices 22 2.8 Summary of approach to relevant matters 23 3 RISK AND THE REGULATORY FRAMEWORK 24 3.1 Background 24 3.2 Revenue risk 27 3.3 Cost risk 33 4 APPORTIONING DAM SAFETY UPGRADE CAPITAL EXPENDITURE 44 4.1 Overview 44 4.2 Dam safety compliance obligations 46 4.3 Recent developments and drivers of dam safety upgrades