Inflammatory Bowel Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SAGES Clinical Spotlight Review: Intraoperative Cholangiography
SAGES Clinical Spotlight Review: Intraoperative cholangiography William W. Hope, MD, Robert Fanelli MD, Danielle S. Walsh MD, Ray Price MD, Dimitrios Stefanidis MD, William S. Richardson MD, and the SAGES Guidelines Committee Preamble The following clinical spotlight review regarding the intraoperative cholangiogram is intended for physicians who manage and treat gallbladder/biliary pathology and perform laparoscopic cholecystectomy. It is meant to critically review the technique of intraoperative cholangiography, alternatives for intraoperative biliary imaging, and the available evidence supporting their safety and efficacy. Based on the level of evidence, recommendations may or may not be given for their use in clinical practice. Disclaimer Guidelines for clinical practice and spotlight reviews are intended to indicate preferable approaches to medical problems as established by experts in the field. These recommendations will be based on existing data or a consensus of expert opinion when little or no data are available. Spotlight reviews are applicable to all physicians who address the clinical problem(s) without regard to specialty training or interests, and are intended to convey recommendations based on a focused topic; within the defined scope of review, they indicate the preferable, but not necessarily the only acceptable approaches due to the complexity of the healthcare environment. Guidelines and recommendations are intended to be flexible. Given the wide range of specifics in any health care problem, the surgeon must always choose the course best suited to the individual patient and the variables in existence at the moment of decision. Guidelines, spotlight reviews, and recommendations are developed under the auspices of the Society of American Gastrointestinal Endoscopic Surgeons and its various committees, and approved by the Board of Governors. -

Impact of Preoperative Endoscopic Ultrasound in Surgical Oncology
REVIEW Impact of preoperative endoscopic ultrasound in surgical oncology Endoscopic ultrasound (EUS) has a strong impact on the imaging and staging of solid tumors within or in close proximity of the upper GI tract. Technological developments during the last two decades have increased the image quality and allowed very detailed visualization of local tumor spread and lymph node affection. Current indications for EUS of the upper GI tract encompass the differentiation between benign and malignant lesions, the staging of esophageal, gastric and pancreatic cancer, and the procurement of a biopsy specimen through fine-needle aspiration. Various technical innovations during the past two decades have increased the diagnostic quality and have simultaneously strengthened the role of EUS in the clinical setting. This article will give a compressed summary on the current state of EUS and possible further technical developments. 1 KEYWORDS: 3D imaging elastosonography endoscopic ultrasound miniprobes Sascha S Chopra & oncologic surgery Michael Hünerbein† 1Department of General & Transplantation Surgery, Charité Campus Virchow-Clinic, Berlin, Conventional endoscopic ultrasound the so-called ‘miniprobes’ into the biliary system Germany Linear versus radial systems or the pancreatic duct in order to obtain high-res- †Author for correspondence: Department of Surgery & Surgical Endoscopic ultrasound (EUS) with flex- olution radial ultrasound images locally. Present Oncology, Helios Hospital Berlin, ible endoscopes is an important diagnostic and mini probes show a diameter of 2–3 mm and oper- 13122 Berlin, Germany Tel.: +49 309 417 1480 therapeutic tool, especially for the local staging ate with frequencies between 12 and 30 MHz. Fax: +49 309 417 1404 of gastrointestinal (GI) cancers, the differen- The main drawbacks of these devices are the lim- michael.huenerbein@ tiation between benign and malignant tumors, ited durability and the decreased depth of penetra- helios-kliniken.de and interventional procedures, such as biopsies tion (~2 cm). -

ACR Manual on Contrast Media
ACR Manual On Contrast Media 2021 ACR Committee on Drugs and Contrast Media Preface 2 ACR Manual on Contrast Media 2021 ACR Committee on Drugs and Contrast Media © Copyright 2021 American College of Radiology ISBN: 978-1-55903-012-0 TABLE OF CONTENTS Topic Page 1. Preface 1 2. Version History 2 3. Introduction 4 4. Patient Selection and Preparation Strategies Before Contrast 5 Medium Administration 5. Fasting Prior to Intravascular Contrast Media Administration 14 6. Safe Injection of Contrast Media 15 7. Extravasation of Contrast Media 18 8. Allergic-Like And Physiologic Reactions to Intravascular 22 Iodinated Contrast Media 9. Contrast Media Warming 29 10. Contrast-Associated Acute Kidney Injury and Contrast 33 Induced Acute Kidney Injury in Adults 11. Metformin 45 12. Contrast Media in Children 48 13. Gastrointestinal (GI) Contrast Media in Adults: Indications and 57 Guidelines 14. ACR–ASNR Position Statement On the Use of Gadolinium 78 Contrast Agents 15. Adverse Reactions To Gadolinium-Based Contrast Media 79 16. Nephrogenic Systemic Fibrosis (NSF) 83 17. Ultrasound Contrast Media 92 18. Treatment of Contrast Reactions 95 19. Administration of Contrast Media to Pregnant or Potentially 97 Pregnant Patients 20. Administration of Contrast Media to Women Who are Breast- 101 Feeding Table 1 – Categories Of Acute Reactions 103 Table 2 – Treatment Of Acute Reactions To Contrast Media In 105 Children Table 3 – Management Of Acute Reactions To Contrast Media In 114 Adults Table 4 – Equipment For Contrast Reaction Kits In Radiology 122 Appendix A – Contrast Media Specifications 124 PREFACE This edition of the ACR Manual on Contrast Media replaces all earlier editions. -

MRCP Vs. ERCP
MRCPMRCP vs.vs. ERCPERCP SteveSteve Harrell,Harrell, MD,MD, MSPHMSPH AdvancedAdvanced TherapeuticTherapeutic EndoscopyEndoscopy DecemberDecember 6,6, 20072007 University of Louisville InitialInitial ThoughtsThoughts ““So,So, itit isis mymy predictionprediction thatthat MRCPMRCP willwill havehave aa hugehuge effecteffect onon ERCPERCP practicepractice inin thethe UnitedUnited States.States.”” ““IfIf II hadhad aa pancreaticpancreatic oror biliarybiliary problemproblem II wouldwould searchsearch outout …… aa centercenter withwith thethe mostmost sophisticatedsophisticated noninvasivenoninvasive techniquestechniques…… veryvery quickly.quickly.”” ““WeWe allall wantwant thethe bestbest forfor ourour patients;patients; shouldshould wewe treattreat themthem differentlydifferently thanthan wewe wouldwould ourselves?ourselves?”” 5/15/985/15/98 Peter B. Cotton, MD, FRCP Medical University of South Carolina Charleston, South Carolina Universityhttp://www.ddc.musc.edu/ddc_pro/pro_development of Louisville /hot_topics/impact_MRCP-cotton.htm LearningLearning GoalsGoals KnowKnow whatwhat ERCPERCP andand MRCPMRCP standstand forfor AdvantagesAdvantages andand disadvantagesdisadvantages ofof MRCPMRCP IndicationsIndications forfor ERCPERCP PoorPoor IndicationsIndications forfor ERCPERCP ClinicalClinical UseUse inin commoncommon disordersdisorders forfor MRCPMRCP EffectsEffects ofof MRCPMRCP onon ERCPERCP inin trainingtraining CasesCases University of Louisville ERCPERCP EndoscopicEndoscopic retrograderetrograde cholangiopancreatographycholangiopancreatography -

Ography C Virtual Colonoscopy for Screening
466 Gut 2004;53:466 Gut: first published as on 11 February 2004. Downloaded from Please visit the Gut website (www.gutjnl.com) for links possible to generate three dimensional ultrasound cholangiograms. to these articles – many to full text. The authors prospectively evaluated the ability of this technique, compared with direct cholangiography (endoscopic retrograde cholangiopancreatography (ERCP)/percutaneous transhepatic cho- langiogram (PTC)) and MRCP, to detect and characterise biliary ....................................................................... obstruction in 40 patients. Experienced operators, who were blinded to the results of the other tests, evaluated images for Pseudo-pseudomembranous collagenous technical adequacy, presence and level of obstruction, and c suspected cause of any stricture. Compared with two dimensional colitis ultrasound, three dimensional analysis improved the assessment of m Yuan S, Reyes V, Bronner MP. Pseudomembranous collagenous colitis. Am J biliary anatomy in seven of 40 patients. Three dimensional Surg Pathol 2003;27:1375–9. ultrasound however visualised the peripapillary region less well Microscopic colitis has been divided into three types (Warren BF, et (80%) than MRCP (95%) and direct cholangiography (100%) but al. Histopathology 2002;40:374–6), all characterised by watery was superior at demonstrating the gall bladder and biliary tree diarrhoea and minimal mucosal changes at colonoscopy, asso- proximal to a stricture. All techniques were highly sensitive for ciated with an increase in lamina propria lymphocytes and minimal detection of biliary obstruction (100%) and each diagnosed the crypt architectural distortion. Of the three types, lymphocytic colitis likely cause in 90–95% of cases. Three dimensional ultrasound also has an increase in intraepithelial lymphocytes, collagenous detected the correct level of obstruction in 92% of cases compared colitis has a subepithelial collagen band, and microscopic colitis not with 95% for MRCP and 90% for ERCP/PTC. -

Diagnosis and Management of Primary Sclerosing Cholangitis
AASLD PRACTICE GUIDELINES Diagnosis and Management of Primary Sclerosing Cholangitis Roger Chapman,1 Johan Fevery,2 Anthony Kalloo,3 David M. Nagorney,4 Kirsten Muri Boberg,5 Benjamin Shneider,6 and Gregory J. Gores7 Preamble classification used by the Grading of Recommendation This guideline has been approved by the American Asso- Assessment, Development, and Evaluation (GRADE) ciation for the Study of Liver Diseases and represents the workgroup with minor modifications (Table 1).3 The position of the Association. These recommendations pro- strength of recommendations in the GRADE system are vide a data-supported approach. They are based on the classified as strong (class 1) or weak (class 2). The quality following: (1) formal review and analysis of the recently- of evidence supporting strong or weak recommendations published world literature on the topic (Medline search); is designated by one of three levels: high (level A), mod- (2) American College of Physicians Manual for Assessing erate (level B), or low-quality (level C). Health Practices and Designing Practice Guidelines1; (3) guideline policies, including the AASLD Policy on the Definition and Diagnosis Development and Use of Practice Guidelines and the Definitions. Primary sclerosing cholangitis (PSC) is a American Gastroenterological Association Policy State- chronic, cholestatic liver disease characterized by inflam- ment on Guidelines2; and (4) the experience of the au- mation and fibrosis of both intrahepatic and extrahepatic thors in the specified topic. bile ducts,4 leading to the formation of multifocal bile Intended for use by physicians, these recommenda- duct strictures. PSC is likely an immune mediated, pro- tions suggest preferred approaches to the diagnostic, ther- gressive disorder that eventually develops into cirrhosis, apeutic and preventative aspects of care. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

Is Intravenous Cholangiography an Alternative to the Routine Per-Operative Cholangiogram? Simon P.J
Postgraduate Medical Journal (1989) 65, 896 - 899 Postgrad Med J: first published as 10.1136/pgmj.65.770.896 on 1 December 1989. Downloaded from Is intravenous cholangiography an alternative to the routine per-operative cholangiogram? Simon P.J. Huddy and John A. Southam Epsom District Hospital, Dorking Road, Epsom, Surrey, UK Summary: Ultrasonography, although an accurate method ofdetecting stones within the gall bladder, is unreliable for the detection of bile duct stones for which per-operative cholangiography remains the standard investigation. Fifty seven patients undergoing elective cholecystectomy had both a pre-operative intravenous cholangiogram and per-operative cholangiography. The pre-operative investigation is shown to be at least as effective in the detection of common bile duct stones and only missed a duct stone in one patient. The substitution of pre-operative intravenous cholangiography for routine per-operative cholangiography would result in a significant reduction in operating time, may provide advance knowledge on the biliary anatomy and would allow advance planning of the likely procedure. It is suggested that pre-operative intravenous cholangiography, carried out on the day of admission, should be considered as a preferred alternative investigation to per-operative cholangiography. Introduction Protected by copyright. Per-operative cholangiography, first introduced by An intravenous cholangiogram was performed as Mirizzi in 1932,' is now a commonly performed an outpatient procedure before admission for procedure. It is considered by many authorities to be cholecystectomy. A control film was taken prior to an investigation which should be routinely performed infusion of 100 ml of meglumine iodoxamate at the time of cholecystectomy in order to identify (Endobil, E. -
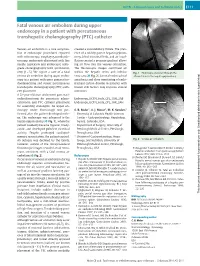
Fatal Venous Air Embolism During Upper Endoscopy in a Patient with Percutaneous Transhepatic Cholangiography (PTC) Catheter
UCTN – Unusual cases and technical notes E111 Fatal venous air embolism during upper endoscopy in a patient with percutaneous transhepatic cholangiography (PTC) catheter Venous air embolism is a rare complica- created a vasculobiliary fistula. The pres- tion of endoscopic procedures reported ence of a widely patent hepaticojejunos- with colonoscopy, esophagogastroduode- tomy, blind intestinal limb, and air insuf- noscopy, endoscopic ultrasound with fine flation created a pressure gradient allow- needle aspiration and endoscopic retro- ing air flow into the venous circulation. grade cholangiography with sphincterot- The fluoroscopic images confirmed air – omy [1 5]. We report a case of a fatal within the hepatic veins and inferior Fig. 1 Endoscope advanced through the venous air embolism during upper endos- vena cava (●" Fig. 2). General endotracheal afferent limb to the hepaticojejunostomy. copy in a patient with prior pancreatico- anesthesia and close monitoring of endo- duodenectomy and recent percutaneous tracheal carbon dioxide in patients with transhepatic cholangiography (PTC) cath- known risk factors may improve clinical eter placement. outcomes. A 52-year-old man underwent pancreati- coduodenectomy for pancreatic adeno- Endoscopy_UCTN_Code_CPL_1AH_2AB carcinoma and PTC catheter placement Endoscopy_UCTN_Code_CPL_1AK_2AH for ascending cholangitis. An upper en- doscopy under fluoroscopy was per- C. B. Meier1, A. J. Moser2, M. K. Sanders3 formed after the patient developed mele- 1 University of Colorado Health Sciences na. The endoscope was advanced to the Center – Gastroenterology, Hepatology, hepaticojejunostomy (●" Fig. 1), when the Aurora, Colorado, USA patient suddenly became hypoxic, brady- 2 Department of Surgery, University of cardic, and developed pulseless electrical Pittsburgh Medical Center, Pittsburgh, activity. Despite prolonged cardiopul- Pennsylvania, USA monary resuscitation, the patient expired. -

Cholescintigraphy Stellingen
M CHOLESCINTIGRAPHY STELLINGEN - • - . • • ' - • i Cholescintigrafie is een non-invasief en betrouwbaar onderzoek in de diagnostiek bij icterische patienten_doch dient desalniettemin als een complementaire en niet als compfititieye studie beschouwd te worden. ]i i Bij de abceptatie voor levensverzekeringen van patienten met ! hypertensie wordt onvoldoende rekening gehouden met de reactie jj op de ingestelde behandeling. ! Ill | Ieder statisch scintigram is een functioneel beeld. | 1 IV ] The purpose of a liver biopsy is not to obtain the maximum \ possible quantity of liver tissue, but to obtain a sufficient 3 quantity with the minimum risk to the patient. j V ( Menghini, 1970 ) I1 Bij post-traumatische verbreding van het mediastinum superius is I angiografisdi onderzoek geindiceerd. VI De diagnostische waarde van een radiologisch of nucleair genees- kundig onderzoek wordt niet alleen bepaald door de kwaliteit van de apparatuur doch voonnamelijk door de deskundigheid van de onderzoeker. VII Ultra sound is whistling in the dark. VIII De opname van arts-assistenten, in opleiding tot specialist, in de C.A.O. van het ziekenhuiswezen is een ramp voor de opleiding. IX De gebruikelijke techniek bij een zogenaamde "highly selective vagotomy" offert meer vagustakken op dan noodzakelijk voor reductie van de zuursecretie. X Het effect van "enhancing" sera op transplantaat overleving is groter wanneer deze sera tijn opgewekt onder azathioprine. XI Gezien de contaminatiegraad van in Nederland verkrijgbare groenten is het gebraik als rauwkost ten stelligste af te raden. j Het het ontstaan van een tweede maligniteit als complicatie van 4 cytostatische therapie bij patienten met non-Hodgkin lymphoma, | maligne granuloom en epitheliale maligne aandoeningen dient, j vooral bij langere overlevingsduur, rekening gehouden te worden. -

OPERATIVE CHOLANGIOGRAPHY by A
388 .. Postgrad Med J: first published as 10.1136/pgmj.32.370.388 on 1 August 1956. Downloaded from OPERATIVE CHOLANGIOGRAPHY By A. W. NURICK, F.R.C.S. Late Senior Registrar, The Middlesex Hospital There is no doubt that stones may be left behind Exploration of the common bile duct does not in the common bile duct after operation. Estimates necessarily mean that it has been completely of the incidence of this complication vary widely cleared of stones and the practice of T tube drain- and the following table shows some of the pub- age of the duct has been generally adopted because lished figures: of the danger of intraperitoneal leakage of bile from the suture line should the duct be closed and INCIDENCE OF RESIDUAL STONE IN THE COMMON DUCT obstruction to the flow of bile into the duodenum Authority Incidence develop. It is customary, before removing a T tube, to Mallet-Guy and Gangolphe (I953) .. 6.9% This Demel (1952) ..... 5.5% perform cholangiography. investigation is Johnston, Waugh and Good (1954) . 8% usually responsible for making the diagnosis of a Buxton and Burk (1948) . .. 5.9% stone in the common duct which has been missed Glenn (I952) ... ... 7% at operation. Post-operative cholangiography is McKittrick and Wilson (1949) .. 2% about io after and whenProtected by copyright. days .. performed operation Bruce (I954) .. 2.5% Hicken, McAllister and Call (I954) . 20% it demonstrates a residual stone the surgeon is Pribram (1947) .. 2.. 1I6-25% presented with information which would have been both more useful and more welcome had it been It is difficult to interpret the higher figures except available while the abdomen was still open at the as a reflection on the frequency and thoroughness original operation. -
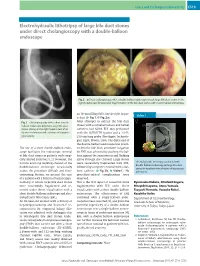
Electrohydraulic Lithotripsy of Large Bile Duct Stones Under Direct Cholangioscopy with a Double-Balloon Endoscope
Cases and Techniques Library (CTL) E519 Electrohydraulic lithotripsy of large bile duct stones under direct cholangioscopy with a double-balloon endoscope Fig. 2 a Direct cholangioscopy with a double-balloon endoscope reveals large bile duct stones in the right hepatic duct. b Successful fragmentation of the bile duct stones with electrohydraulic lithotripsy. an 18-mm filling defect in the right hepat- Video 1 ic duct (●" Fig. 1,●" Fig.2a). Fig. 1 Cholangiography with a short double- After attempts to extract the bile duct balloon endoscope delineates large bile duct stones with a retrieval balloon and basket stones (arrow) in the right hepatic duct of an catheters had failed, EHL was performed 82-year-old woman with a history of hepatico- with the AUTOLITH System and a 1.9-Fr, jejunostomy. 250-cm-long probe (Northgate Technolo- gies, Elgin, Illinois, USA). The distal end of the double-balloon endoscope was insert- The use of a short double-balloon endo- ed into the bile duct, and water irrigation scope facilitates the endoscopic removal for EHL was achieved by pushing the bal- of bile duct stones in patients with surgi- loon against the anastomosis and flushing cally altered anatomy [1,2]. However, the saline through the channel. Large stones narrow and long working channel of the were successfully fragmented with EHL, Electrohydraulic lithotripsy combined with double-balloon endoscopy for large bile duct double-balloon endoscope occasionally followed by complete removal with a bal- stones in a patient with a history of hepaticoje- ●" ●" makes the procedure difficult and time- loon catheter ( Fig. 2b, Video1).