Research Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
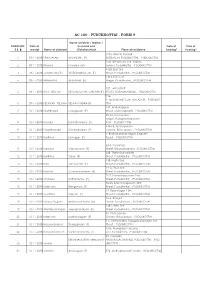
Ac 180 - Pudukkottai - Form 9
AC 180 - PUDUKKOTTAI - FORM 9 Name of Father / Mother / ANNEXURE Date of Husband and Date of Time of 5.8 receipt Name of claimant (Relationship)# Place of residence hearing* hearing* 3774, ASHOK NAGAR 1 09-11-2018 SRINIVASAN BALAGURU (F) EXTENSION, PUDUKKOTTAI, , PUDUKKOTTAI Old 129 new no 114 , Nizam 2 09-11-2018 Bharani Manjula (M) colony, Pudukkottai, , PUDUKKOTTAI 4428, East 3rd 3 09-11-2018 JAYAGOMATHI VIVEKANANDHAN (F) Street, Pudukkottai, , PUDUKKOTTAI 1343, Kamban 4 09-11-2018 BHAARATH RETHINAM (F) Nagar, Pudukkottai, , PUDUKKOTTAI 727 , MALAIYUR 5 09-11-2018 SIVA SELVAM SELVAM DHANALAKSHMI (F) SOUTH, KARAMBAKKUDI, , PUDUKKOTTAI 224- 1, MURUNKAKOLLAI, MALAIYUR, , PUDUKKO 6 09-11-2018 KESAVAN SELVAM SELVAM INDRA (F) TTAI 147, Ambalagarar 7 10-11-2018 Sountharya Murugesan (F) Street, Maravappatti, , PUDUKKOTTAI 98-3B, Ganapathi Nagar, Rajagopalapuram 8 10-11-2018 Monika Kulanthaisamy (F) Post, , PUDUKKOTTAI 414-18, Ambalpuram 9 10-11-2018 Priyadharshini Ravichandran (F) Colony, Thiruvappur, , PUDUKKOTTAI 1, Brahadambal Nagar, Koilpatti 10 10-11-2018 Karthika Murugan (F) Road, , PUDUKKOTTAI 40-3, Yanaimal 11 10-11-2018 Saranya Vijayakumar (F) Street, Thirukkokarnam, , PUDUKKOTTAI 23B, Thetchinamoorthi 12 10-11-2018 Karthika Sekar (F) Street, Pudukkottai, , PUDUKKOTTAI 158, North 2nd 13 10-11-2018 Raja Tamilselvan (F) Street, Pudukkottai, , PUDUKKOTTAI 1416, West 3rd 14 10-11-2018 Kavitha Chandrasekaran (F) Street, Pudukkottai, , PUDUKKOTTAI 2-17, Kamarajapuram 2nd 15 10-11-2018 Umadevi Kathamuthu (F) Street, Pudukkottai, , PUDUKKOTTAI -

District Census Handbook, Pudukkottai, Part XII a & B, Series-23
CENSUS OF INDIA 1991 SERIES - 23 TAMIL NADU DISTRICT CENSUS HANDBOOK PUDUKKOlTAI PARTXII A&B VILLAGE AND TOWN DIRECTORY VILLAGE AND TOWNWISE PRIMARY CENSUS ABSTRACT K. SAMPATH KUMAR OF THE INDIAN ADMINISTRATIVE SERVICE DIRECTOR OF CENSUS OPERATIONS TAMILNADU CONTENTS Pag,~ No. 1. Foreward (vii-ix) 2. Preface (xi-xv) 3. Di::'trict Map Facing Page .:;. Important Statistics 1-2 5. Analytical Note: I) Census concepts: Rural and Urban areas, Urban Agglomeration, Census House/Household, SC/ST, Literates, Main Workers, Marginal 3-4 Workers, Non-Workers etc. H) History of the District Census Handhook including scope of Village and Town Directory and Primary Census Abstract. 5-9 iii) History of the District and its Formation, Location and Physiography, Forestry, Flora and Fauna, Hills, Soil, Minerals and Mining, Rivers, EledricHy and Power, Land and Land use pattern, Agriculture and Plantations, Irrigation, Animal Husbandry, Fisheries, Industries, Trade and Commerce, Transpoli and Communications, Post and Telegraph, Rainfall, Climate and Temperature, Education, People, Temples and Places of Tourist Importance. lO-20 6. Brief analysis of the Village and Town Dirctory and Primary Census Abstract data. 21-41 PART-A VILLAGE AND TOWN DIRECTORY Section-I Village Directory 43 Note explaining the codes used in the Village Directory. 45 1. Kunnandarkoil C.D. Block 47 i) Alphabetical list of villages 48-49 ii) Village Directory Statement 50-55 2. Annavasal C.D. Block 57 i) Alphabetical list of villages 50-59 iil Village Directory Statement 60-67 3. Viralimalai C.D. Block 69 i) Alphabetical list of villages 70-71 iil Village Directory Statement 72-79 4. -
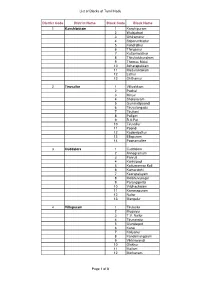
List of Blocks of Tamil Nadu District Code District Name Block Code
List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 1 Kanchipuram 1 Kanchipuram 2 Walajabad 3 Uthiramerur 4 Sriperumbudur 5 Kundrathur 6 Thiruporur 7 Kattankolathur 8 Thirukalukundram 9 Thomas Malai 10 Acharapakkam 11 Madurantakam 12 Lathur 13 Chithamur 2 Tiruvallur 1 Villivakkam 2 Puzhal 3 Minjur 4 Sholavaram 5 Gummidipoondi 6 Tiruvalangadu 7 Tiruttani 8 Pallipet 9 R.K.Pet 10 Tiruvallur 11 Poondi 12 Kadambathur 13 Ellapuram 14 Poonamallee 3 Cuddalore 1 Cuddalore 2 Annagramam 3 Panruti 4 Kurinjipadi 5 Kattumannar Koil 6 Kumaratchi 7 Keerapalayam 8 Melbhuvanagiri 9 Parangipettai 10 Vridhachalam 11 Kammapuram 12 Nallur 13 Mangalur 4 Villupuram 1 Tirukoilur 2 Mugaiyur 3 T.V. Nallur 4 Tirunavalur 5 Ulundurpet 6 Kanai 7 Koliyanur 8 Kandamangalam 9 Vikkiravandi 10 Olakkur 11 Mailam 12 Merkanam Page 1 of 8 List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 13 Vanur 14 Gingee 15 Vallam 16 Melmalayanur 17 Kallakurichi 18 Chinnasalem 19 Rishivandiyam 20 Sankarapuram 21 Thiyagadurgam 22 Kalrayan Hills 5 Vellore 1 Vellore 2 Kaniyambadi 3 Anaicut 4 Madhanur 5 Katpadi 6 K.V. Kuppam 7 Gudiyatham 8 Pernambet 9 Walajah 10 Sholinghur 11 Arakonam 12 Nemili 13 Kaveripakkam 14 Arcot 15 Thimiri 16 Thirupathur 17 Jolarpet 18 Kandhili 19 Natrampalli 20 Alangayam 6 Tiruvannamalai 1 Tiruvannamalai 2 Kilpennathur 3 Thurinjapuram 4 Polur 5 Kalasapakkam 6 Chetpet 7 Chengam 8 Pudupalayam 9 Thandrampet 10 Jawadumalai 11 Cheyyar 12 Anakkavoor 13 Vembakkam 14 Vandavasi 15 Thellar 16 Peranamallur 17 Arni 18 West Arni 7 Salem 1 Salem 2 Veerapandy 3 Panamarathupatti 4 Ayothiyapattinam Page 2 of 8 List of Blocks of Tamil Nadu District Code District Name Block Code Block Name 5 Valapady 6 Yercaud 7 P.N.Palayam 8 Attur 9 Gangavalli 10 Thalaivasal 11 Kolathur 12 Nangavalli 13 Mecheri 14 Omalur 15 Tharamangalam 16 Kadayampatti 17 Sankari 18 Idappady 19 Konganapuram 20 Mac. -
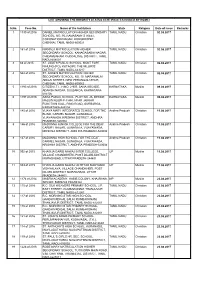
S.No. Case No. Name of the Institution State Religion Date of Issue
LIST SHOWING THE MINORITY STATUS CERTIFICATES ISSUED BY NCMEI S.No. Case No. Name of the Institution State Religion Date of Issue Remarks 1 1330 of 2016 DANIEL MATRICULATION HIGHER SECONDARY TAMIL NADU Christian 02.08.2017 SCHOOL, NO. 36, RAMASAMY STREET, CORONATION NAGAR, KORUKKUPET, CHENNAI, TAMIL NADU-600021 2 161 of 2016 NIRMALA MATRICULATION HIGHER TAMIL NADU Christian 02.08.2017 SECONDARY SCHOOL, KANAGASABAI NAGAR, CHIDAMBARAM, CUDDALORE DISTRICT, TAMIL NADU-608001 3 68 of 2015 ST. JUDE’S PUBLIC SCHOOL, MONT FORT, TAMIL NADU Christian 02.08.2017 NIHUNG (PO), KOTAGIRI, THE NILGIRIS DISTRICT, TAMIL NADU-643217 4 544 of 2016 ST. ANNE’S MATRICULATION HIGHER TAMIL NADU Christian 02.08.2017 SECONDARY SCHOOL, NO. 10, MARAIMALAI ADIGAI STREET, NEW PERUNGALATHUR, CHENNAI, TAMIL NADU-600063 5 1193 of 2016 CITIZEN I.T.I., H.NO. 2-907, SANA ARCADE, KARNATAKA Muslim 09.08.2017 ADARSH NAGAR, GULBARGA, KARNATAKA- 585105 6 1197 of 2016 ASRA PUBLIC SCHOOL, PLOT NO. 26, BESIDE KARNATAKA Muslim 09.08.2017 MASJID NOOR-E-ILAHI, NEAR JABBAR FUNCTION HALL, RING ROAD, GURBARGA, KARNATAKA-585104 7 145 of 2016 VIJAYA MARY INTEGRATED SCHOOL FOR THE Andhra Pradesh Christian 11.08.2017 BLIND, CARMEL NAGAR, GUNADALA, VIJAYAWADA, KRISHNA DISTRICT, ANDHRA PRADESH-520004 8 146 of 2016 MADONNA JUNIOR COLLEGE FOR THE DEAF, Andhra Pradesh Christian 11.08.2017 CARMEL NAGAR, GUNADALA, VIJAYAWADA, KRISHNA DISTRICT, ANDHRA PRADESH-520004 9 147 of 2016 MADONNA HIGH SCHOOL FOR THE DEAF, Andhra Pradesh Christian 11.08.2017 CARMEL NAGAR, GUNADALA, VIJAYAWADA, KRISHNA DISTRICT, ANDHRA PRADESH-520004 10 992 of 2015 KHAWJA GARIB NAWAJ INTER COLLEGE, UP Muslim 11.08.2017 VILLAGE CHANDKHERI, POST DILARI, DISTRICT MORADABAD, UTTAR PRADESH-244401 11 993 of 2015 KHAWJA GARIB NAWAJ UCHTTAR MADYAMIK UP Muslim 11.08.2017 VIDHYALAYA, VILLAGE CHANDKHERI, POST DILARI, DISTRICT MORADABAD, UTTAR PRADESH-244401 12 1174 of 2014 MABFM ACADEMY, HABIB COLONY, KHAJRANA, MP Muslim 23.08.2017 INDORE, MADHYA PRADESH 13 115 of 2016 R.C. -

Mint Building S.O Chennai TAMIL NADU
pincode officename districtname statename 600001 Flower Bazaar S.O Chennai TAMIL NADU 600001 Chennai G.P.O. Chennai TAMIL NADU 600001 Govt Stanley Hospital S.O Chennai TAMIL NADU 600001 Mannady S.O (Chennai) Chennai TAMIL NADU 600001 Mint Building S.O Chennai TAMIL NADU 600001 Sowcarpet S.O Chennai TAMIL NADU 600002 Anna Road H.O Chennai TAMIL NADU 600002 Chintadripet S.O Chennai TAMIL NADU 600002 Madras Electricity System S.O Chennai TAMIL NADU 600003 Park Town H.O Chennai TAMIL NADU 600003 Edapalayam S.O Chennai TAMIL NADU 600003 Madras Medical College S.O Chennai TAMIL NADU 600003 Ripon Buildings S.O Chennai TAMIL NADU 600004 Mandaveli S.O Chennai TAMIL NADU 600004 Vivekananda College Madras S.O Chennai TAMIL NADU 600004 Mylapore H.O Chennai TAMIL NADU 600005 Tiruvallikkeni S.O Chennai TAMIL NADU 600005 Chepauk S.O Chennai TAMIL NADU 600005 Madras University S.O Chennai TAMIL NADU 600005 Parthasarathy Koil S.O Chennai TAMIL NADU 600006 Greams Road S.O Chennai TAMIL NADU 600006 DPI S.O Chennai TAMIL NADU 600006 Shastri Bhavan S.O Chennai TAMIL NADU 600006 Teynampet West S.O Chennai TAMIL NADU 600007 Vepery S.O Chennai TAMIL NADU 600008 Ethiraj Salai S.O Chennai TAMIL NADU 600008 Egmore S.O Chennai TAMIL NADU 600008 Egmore ND S.O Chennai TAMIL NADU 600009 Fort St George S.O Chennai TAMIL NADU 600010 Kilpauk S.O Chennai TAMIL NADU 600010 Kilpauk Medical College S.O Chennai TAMIL NADU 600011 Perambur S.O Chennai TAMIL NADU 600011 Perambur North S.O Chennai TAMIL NADU 600011 Sembiam S.O Chennai TAMIL NADU 600012 Perambur Barracks S.O Chennai -

Tamil Nadu Public Service Commission Bulletin
© [Regd. No. TN/CCN-466/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 196/2009 2015 [Price: Rs. 280.80 Paise. TAMIL NADU PUBLIC SERVICE COMMISSION BULLETIN No. 18] CHENNAI, SUNDAY, AUGUST 16, 2015 Aadi 31, Manmadha, Thiruvalluvar Aandu-2046 CONTENTS DEPARTMENTAL TESTS—RESULTS, MAY 2015 Name of the Tests and Code Numbers Pages. Pages. Second Class Language Test (Full Test) Part ‘A’ The Tamil Nadu Wakf Board Department Test First Written Examination and Viva Voce Parts ‘B’ ‘C’ Paper Detailed Application (With Books) (Test 2425-2434 and ‘D’ (Test Code No. 001) .. .. .. Code No. 113) .. .. .. .. 2661 Second Class Language Test Part ‘D’ only Viva Departmental Test in the Manual of the Firemanship Voce (Test Code No. 209) .. .. .. 2434-2435 for Officers of the Tamil Nadu Fire Service First Paper & Second Paper (Without Books) Third Class Language Test - Hindi (Viva Voce) (Test Code No. 008 & 021) .. .. .. (Test Code 210), Kannada (Viva Voce) 2661 (Test Code 211), Malayalam (Viva Voce) (Test The Agricultural Department Test for Members of Code 212), Tamil (Viva Voce) (Test Code 213), the Tamil Nadu Ministerial Service in the Telegu (Viva Voce) (Test Code 214), Urdu (Viva Agriculture Department (With Books) Test Voce) (Test Code 215) .. .. .. 2435-2436 Code No. 197) .. .. .. .. 2662-2664 The Account Test for Subordinate Officers - Panchayat Development Account Test (With Part-I (With Books) (Test Code No. 176) .. 2437-2592 Books) (Test Code No. 202).. .. .. 2664-2673 The Account Test for Subordinate Officers The Agricultural Department Test for the Technical Part II (With Books) (Test Code No. 190) .. 2593-2626 Officers of the Agriculture Department Departmental Test for Rural Welfare Officer (With Books) (Test Code No. -

List of Colleges
BHARATHIDASAN UNIVERSITY TIRUCHIRAPPALLI – 24 NATIONAL SERVICE SCHEME CELL List of NSS Functioning Colleges for the year 2019-20 with Units (Aided & SF) No. of Units S.No. Name of the College Aided SF Total Tiruchirappalli District 1. Bharathidasan University (Departments), Trichy – 620024 2 2 2. Bishop Heber College (Autonomous), Trichy – 620 017 5 1 6 3. Cauvery College for Women, Trichy - 620 018 5 5 4. Chidambaram Pillai College for Women, Trichy - 621005 5 5 5. Christhu Raj College, Trichy – 620 012 1 1 6. Govt. Arts College, Trichy-620 022 3 3 7. Holy Cross College (Autonomous), Trichy- 620 002 5 5 8. Jamal Mohamed College (Autonomous), Trichy – 620 020 7 7 9. Kurinji College of Arts & Science, Trichy - 620 002 2 2 10. M.I.E.T. Arts & Science College, Trichy – 620 007 3 3 11. National College (Autonomous), Trichy – 620 001 4 4 Nehru Memorial College(Autonomous), Puthanampatti - 12. 5 5 621007 13. Periyar EVR College (Autonomous), Trichy -620 023 4 4 14. Seethalakshmi Ramaswamy College, Trichy -620 002 5 5 15. Shrimati Indira Gandhi College, Trichy - 620 002 5 5 16. Srimad Andavan Arts & Science College, Trichy - 620005 4 4 17. St. Joseph’s College (Autonomous), Trichy - 620002 5 1 6 18. Urumu Dhanalakshmi College, Trichy -620019 4 4 Aiman College of Arts & Science for Women, Trichy - 19. 2 2 620021 20. Arignar Anna Govt. Arts College, Musiri - 621201 5 5 Imayam Arts and Science College, Kannanur, Thuraiyur- 21. 3 3 621206 22. Govt. Arts & Science College, Kumulur, Lalgudi-621 601 1 1 Bharathidasan University Constituent College, Navalur 23. -

Local Library Authority, Pudukkottai
LOCAL LIBRARY AUTHORITY, PUDUKKOTTAI District Central Library/Branch Libraries Address 8. Librarian, 1. Librarian, 15. Librarian, Branch Library, Kadiyappatti, District Central Library, Branch Library, Narchandupatti, Kadaiveethi, T.S.No.104, West 4th Street, New No.4/41, Perumalkoil Street, Ramachandhrapuram, Pudukkottai - 622 001. Thirumayam(Taluk), Thirumayam(Taluk) Narchanduppatti - 622 404. Kadiyappatti - 622 505. 9. Librarian, 16. Librarian, 2. Librarian, Branch Library, Kulipirai, Branch Library, Branch Library, Aranthangi New No.9-23, Bus Stand, Subramaniyapuram, New Periyapallivasal Street, Ponnamaravathy Main Road, No.2/312, Peravurai Road, Aranthangi - 614 616. Thirumayam(Taluk), Aranthangi(Taluk), Kulipirai- 622 402 Subramaniyapuram - 614 805. 3. Librarian, 10. Librarian, 17. Librarian, Branch Library, Arasarkulam Branch Library, Keeranur, Branch Library, Vadakadu, New No.2/343, North Ratha Street, No.6/1731, Santhaipettai, 143 Chinnapallivasal Street, Kulathur(Taluk), Alangudi(Taluk) Aranthangi(Taluk), Keeranur - 622 505. Vadakadu - 622 304. Arasarkulam - 614 801. 4. Librarian, 11. Librarian, 18. Librarian, Branch Library, Annavasal, Branch Library, Branch Library, Viralimalai, New No.35, Keeramangalam, New No.1291, Santhaipattai, Sannathi Street, Padayal Road, Madurai Main Road, Illuppure(Taluk), Aranthangi(Taluk), Illuppure(Taluk), Annavasal - 622 101. Keeramangalam - 614 624. Viralimalai - 621 316. 5. Librarian, 12. Librarian, Branch Library, Arimalam, Branch Library, 19. Librarian, New No.17, Kothamangalam, Branch Library, Alangudi, Sivankoil Street, Thirumayam New No.5/347, Vadima Nagar, Santhaipettai, Alangudi(Taluk), Arimalam - 622 201. Alangudi(Taluk), Alangudi - 622 301. Kothamangalam - 614 624. 6. Librarian, 13. Librarian, 20. Librarian, Branch Library, Illuppur, New Branch Library, Branch Library, Vendhanpatti, Door No.8, Ponnamaravathy, Nerkuppai Main Road, Kottai Street, New Door No.9, Sivankoil, Thirumayam(Taluk), Pudukkottai Main Road, Ponnamaravathy(Taluk), Vendhanpatti - 622 419. -

List of Treasuries /Sub Treasuries with Code
LIST OF TREASURIES/SUB TREASURIES AGRICULTURAL AEW AGRICULTURAL ENGINEERING WORKS ENGINEERING WORKS ARIYALUR ARL ARIYALUR ARI ARIYALUR JAY JAYANKONDACHOLAPURAM SEN SENDURAI CHENGLEPET- CGT CHENGLEPET-KANCHEEPURAM KANCHEEPURAM ALN ALANDUR ALR WALAJABAD CGP CHINGLEPUT CHR CHEYYUR MDK MADURANTAKAM MML MAMALLAN NAGAR/KANCHEEPURAM ST SNR SHOLINGANALLUR SPR SRIPERUMBUDUR TAM TAMBARAM TNR TIRUKKALIKUNDRAM TRO TIRUPORUR UMR UTHIRAMERUR WBD WALAJABAD CHENNAI MDS CHENNAI EGM EGMORE, NUNGAMBAKKAM MYL MYLAPORE, TRIPLICANE PRB PERAMBUR, PURUSAWAKKAM SDT SAIDAPET, MAMBALAM, GUINDY COIMBATORE CBE COIMBATORE ANR ANNUR CBN COIMBATORE(NORTH)/TATABAD GNP COIMBATORE NORTH/GANAPATHY KNK KINATHUKADAVU MET METTUPALAYAM POL POLLACHI SCB COIMBATORE SOUTH SLR SULUR TOWN TTB TATABAD(COIMBATORE) VPR VALPARAI CUDDALORE VAL CUDDALORE CHD CHIDAMBARAM CUD TIRUPPAPULIYUR/ CUDDALORE ST KNJ KURUNJIPADI KTK KATTUMANNAKOIL NEY NEYVELI PNT PANRUTTI TIT TITTAGUDI VRC VRIDHACHALAM LIST OF TREASURIES/SUB TREASURIES DHARMAPURI DMP DHARMAPURI DMT DHARMAPURI TOWN/DHARMAPURI ST HAR HARUR PAP PAPPIREDDIPATTY/ KOZHIMEKKANUR/MOLIANUR PLC PALACODE PNG RMY PENNAGARAM DINDIGUL DGL DINDIGUL ATH ATHOOR KDK KODAIKANAL NHR NEHRUJI NAGAR, DGL/DINDIGUL ST NLK NILAKOTTAI NTM NATHAM ODN ODDANCHATHRAM PLN PALANI VDS VEDASANDUR ERODE PRY ERODE ANR ANTHIYUR BVN BHAVANI ERD ERODE GBP GOBICHETTIPALAYAM KDM KODUMUDI ADB PRN PERUNDURAI SMG SATHYAMANGALAM IB PAPPIREDDIPATTY/ PAP PAO PENSION KOZHIMEKKANUR/MOLI ANUR KANYAKUMARI KKM KANYAKUMARI BPT THOVALAI/BHOOTHAPANDY BTP BHOOTHAPANDY KLK KALKULAM/THUCKALAY -

I. Profile of Pudukkottai District
I. PROFILE OF PUDUKKOTTAI DISTRICT 1. INTRODUCTION Pudukkottai has a familiar Historical background and it was formerly a Princely State with the title of “SAMASTHANAM” ruled by the “H.H.The Rajah’s of THONDAIMANS”. The present Pudukkottai district is encompassing the entire Princely State of Pudukkottai and parts of Tiruchirappalli and Thanjavur districts. Pudukkottai district came into existence on 14.1.1974. The erstwhile “Pudukkottai State” has been justly famous for its efficient and stable administration through the years with its seasoned administrative system, operating with well understood concepts of hierarchy line of command and discreet adherences to principles and procedures. Really this credit goes to the initial author and as well as the founder of the system of “District Office Manual”, by “Sir Alexander Loftus Tottenham”, the Agent of the British Emperor/ Administrator of erstwhile “Pudukkottai State” for his aim of trim and efficient administration. Pudukkotai District is bounded on the North East and East by Thanjavur District, on the South East by Bay of Bengal, on the South West by Ramanathapuram and Sivaganga districts and on the West and North East by Thiruchirapalli District. 1 2. DISASTER MANAGEMENT PLAN The main objective of Disaster Management Plan is to assess the vulnerability of district to various major hazards so that mitigate steps can be taken to contain the damages before and during disaster and to provide relief and take reconstruction measures at the shortest possible time effectively. The District Disaster Management Plan is also a purposeful document that assigns responsibility to the officials of Government Departments, Social Organisations and Individuals for carrying out specific and effective actions at projected times and places in an emergency manner that exceeds the capability or routine responsibility of an one agency, e.g 2 the departments of Revenue, Police, Fire Services, Fisheries, Highways, PWD, South Vellar Division and Health etc. -
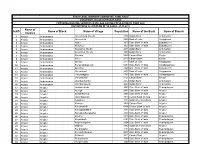
S.NO Name of District Name of Block Name of Village Population Name
STATE LEVEL BANKERS' COMMITTEE, TAMIL NADU CONVENOR: INDIAN OVERSEAS BANK PROVIDING BANKING SERVICES IN VILLAGE HAVING POPULATION OF OVER 2000 DISTRICTWISE ALLOCATION OF VILLAGES -01.11.2011 Name of S.NO Name of Block Name of Village Population Name of the Bank Name of Branch District 1 Ariyalur Andiamadam Anikudichan (South) 2730 Indian Bank Andimadam 2 Ariyalur Andiamadam Athukurichi 5540 Bank of India Alagapuram 3 Ariyalur Andiamadam Ayyur 3619 State Bank of India Edayakurichi 4 Ariyalur Andiamadam Kodukkur 3023 State Bank of India Edayakurichi 5 Ariyalur Andiamadam Koovathur (North) 2491 Indian Bank Andimadam 6 Ariyalur Andiamadam Koovathur (South) 3909 Indian Bank Andimadam 7 Ariyalur Andiamadam Marudur 5520 Canara Bank Elaiyur 8 Ariyalur Andiamadam Melur 2318 Canara Bank Elaiyur 9 Ariyalur Andiamadam Olaiyur 2717 Bank of India Alagapuram 10 Ariyalur Andiamadam Periakrishnapuram 5053 State Bank of India Varadarajanpet 11 Ariyalur Andiamadam Silumbur 2660 State Bank of India Edayakurichi 12 Ariyalur Andiamadam Siluvaicheri 2277 Bank of India Alagapuram 13 Ariyalur Andiamadam Thirukalappur 4785 State Bank of India Varadarajanpet 14 Ariyalur Andiamadam Variyankaval 4125 Canara Bank Elaiyur 15 Ariyalur Andiamadam Vilandai (North) 2012 Indian Bank Andimadam 16 Ariyalur Andiamadam Vilandai (South) 9663 Indian Bank Andimadam 17 Ariyalur Ariyalur Andipattakadu 3083 State Bank of India Reddipalayam 18 Ariyalur Ariyalur Arungal 2868 State Bank of India Ariyalur 19 Ariyalur Ariyalur Edayathankudi 2008 State Bank of India Ariyalur 20 Ariyalur -

Sittannavasal
I SITSITTTANNAANNAVVASALASAL *UN*edited version ASudharsanam centre for Arts and Culture 2 Palace nagar, Pudukkottai I -1- CONTENTS 1. Introduction------------------------------------------ 5 -Approach ----------------------------------- 7 2. Historical Background ------------------------------ 9 3. The Monuments ----------------------------------- 10 -Reaching the monuments --------------- 12 4. The Arivar Koil ------------------------------------- 13 -The Sittannavasal painting -------------- 15 -The Samava-sarana in Jaina tradition -- 16 -The discovery ----------------------------- 17 -The painting tradition ------------------- 18 -The cave temple - A general description ----------------- 19 -The pillared veranda --------------------- 21 -The ardha-mandapam ------------------- 22 -The garbha-griham ----------------------- 32 -Tamil inscriptions near the cave temple -------------------------- 34 5. The Ezhadippattam -------------------------------- 35 6. The Navachchunai --------------------------------- 38 7. The Megalithic Burial Sites ----------------------- 39 8. The Other Interesting Sites ----------------------- 43 Appendix 1. The Pandya inscription ---------------------------- 44 1. INTRODUCTION Sittannavasal† is a small village in Pudukkottai† district and is world famous for its fresco paintings in the rock-cut Jaina cave temple. Sittannavasal† has an important place in the history of Tamilnadu. The megalithic monuments such as stone-circles, urn burials and cists spread in the plains of this village testify that this area was