Uhm Phd 9604137 R.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
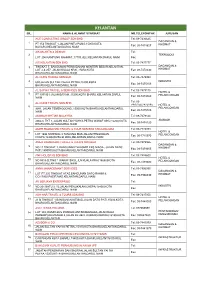
Kelantan Bil
KELANTAN BIL. NAMA & ALAMAT SYARIKAT NO.TELEFON/FAX JURUSAN ACE CONSULTING GROUP SDN BHD Tel: 09-7436625 DAGANGAN & 1 PT 153 TINGKAT 1,JALAN PINTU PONG,15000,KOTA Fax: 09-7418827 KHIDMAT BAHARU,KELANTAN,DARUL NAIM AIKON ARTS & DESIGN Tel: 2 TEKNOLOGI LOT 206 KAMPUNG RAHMAT,,17700,JELI,KELANTAN,DARUL NAIM Fax: AIR KELANTAN SDN BHD Tel: 09-7437777 DAGANGAN & 3 TINGKAT 5, BANGUNAN PERBADANAN MENTERI BESAR,KELANTAN, LOT 2 & 257, JALAN KUALA KRAI,,15050,KOTA Fax: 09-7472030 KHIDMAT BHARU,KELANTAN,DARUL NAIM AL QUDS TRAVEL SDN BHD Tel: 09-7479999 4 650,JALAN SULTAN YAHYA PETRA,15200,KOTA INDUSTRI Fax: 09-7475105 BHARU,KELANTAN,DARUL NAIM AL SAFWA TRAVEL & SERVICES SDN BHD Tel: 09-7475115 HOTEL & 5 PT 1971-B1 JALAN BAYAM,,15200,KOTA BHARU,KELANTAN,DARUL Fax: 09-7479060 PELANCONGAN NAIM Tel: 09- AL-QUDS TRAVEL SDN BHD 7475155/7475145 HOTEL & 6 9981, JALAN TEMENGGONG,,15000,KOTA BHARU,KELANTAN,DARUL PELANCONGAN Fax: 09-7475105 NAIM AMANAH IKHTIAR MALAYSIA Tel: 09-7478124 7 2002-C TKT 1,,JALAN SULTAN YAHYA PETRA WAKAF SIKU,15200,KOTA AMANAH Fax: 09-7478120 BHARU,KELANTAN,DARUL NAIM AMER RAMADHAN TRAVEL & TOUR SDN BHD TANJUNG MAS Tel: 09-7715973 HOTEL & 8 LOT 1894 SIMPANG 3 TANJUNG MAS,JALAN PENGKALAN Fax: 09-7715970 PELANCONGAN CHEPA,15300,KOTA BHARU,KELANTAN,DARUL NAIM AMER RAMADHAN TRAVEL & TOURS SDN BHD Tel: 09-7479966 DAGANGAN & 9 NO 11 TINGKAT 1, BANGUNAN TH,KOMPLEKS NIAGA , JALAN DATO' Fax: 09-7479955 KHIDMAT PATI,1500000,KOTA BHARU,KELANTAN,DARUL NAIM ANF HOLIDAYS SDN BHD Tel: 09-7488600 HOTEL & 10 NO 5515-D,TING 1 WAKAF SIKU,,JLN KUALA -

Public Involvement on Environment Issues in Kota Bharu and Jeli District, Kelantan
Journal of Social Sciences 7 (2): 175-181, 2011 ISSN 1549-3652 © 2010 Science Publications Public Involvement on Environment Issues in Kota Bharu and Jeli District, Kelantan Mohammad Ghazi Ismail and Haliza Abdul Rahman Environmental and Occupational Health Program, School of Health Sciences, University of Science Malaysia, 16150 Kubang Kerian, Kelantan Abstract: Problem statement: Environmental problems are too serious and complex to be solved through scientific approaches, technical and purely legal. Thus, public involvement with a more comprehensive vital is needed. This is because as one of the groups interested, this group can influenced and changed decision related policy legislation and policy related to environment. Public Involvement is needed in every development process as one of the positive move and proactive to create sustainable development. This study carried out in Kota Bharu and Jeli district, Kelantan, Malaysia with 390 respondent involved in each of them. Approach: Major method of study was used investigation question form. This study compared the extent to which a significant correlation (p), Mean (M) and Standard Deviation (SD) of the studied demographic factors as gender, age, race, religion, income, education and the respondent lived with the knowledge of environmental issues, environmental local environment and constraints factors involved in environmental issues at the research areas. Chi-square test used to study demographic factor association with environment issue knowledge respondent. Results: Comparison of demographic factors with knowledge of the respondents of research areas on environmental issues shows that there is relationship for respondents education level with their respective p values is 0.036 and 0.040. The relationship between demographic factors with knowledge of the local environment issues by respondent shows there is also relationship to income and education with their respective p values is 0.033, 0.019 for Kota Bharu and 0014, 0019 for Jeli. -

Branches/Self-Service Machines in Flood Affected Areas
ATTACHMENT (COMMERCIAL BANKS) BRANCHES/SELF-SERVICE MACHINES IN FLOOD AFFECTED AREAS Note: ATM - Automated Teller Machine CRM - Cash Recycling Machine CDM - Cash Deposit Machine CQM - Cheque Deposit Machine Kelantan Functioning ATM/CDM Location/Address of Branch Town Location/Address of Branch No Bank (Incapacitated and not in operation) Location/Address 1 Affin Bank Berhad NO 3788 H–I Jalan Sultan Ibrahim 15050 Kota Bharu, - Branch Kelantan Kota Bharu Kem Desa Pahlawan. 16500, - Offsite Kota Bharu, Kelantan A1 & A2, Block A, Bandar Baru Bukit Bunga, 17700 Jeli Bukit Bunga, Tanah Merah, - Branch Kelantan 2 Alliance Bank Malaysia - - - - Berhad Page 1 of 25 BRANCHES/SELF-SERVICE MACHINES IN FLOOD AFFECTED AREAS Note: ATM - Automated Teller Machine CRM - Cash Recycling Machine CDM - Cash Deposit Machine CQM - Cheque Deposit Machine Kelantan Functioning ATM/CDM Location/Address of Branch Town Location/Address of Branch No Bank (Incapacitated and not in operation) Location/Address 3 AmBank (M) Berhad Branch Branch 13 ATMs at 7 Eleven outlets:- 1) Jalan Raja Perempuan Zainab 2 2) Jalan Tok Kenali 3) Tanjung Chat Ground & First Floor, Lot 343 4) Pasir Tumboh Section 13, Jalan Sultan 5) Kok Lanas Kota Bharu - Ibrahim, 15000 Kota Bharu, 6) Pasir Pekan Offsite Kelantan 7) Taman Muda Murni 8) Wakaf Baru 9) Padang Tembak 10) Kota Jemba 11) Panji 12) Jalan Hospital 13) Pantai Cahaya Bulan 1 ATM at Adventa, Pengkalan Chepa Offsite 1 ATM at Tesco Kota Bahru 1 ATM at Mydin Kubang Lot 151, Jalan Masjid Lama, Pasir Mas - Branch 17000 Pasir Mas, Kelantan -

Concern of Veterinary Authorities with Respect to Borders Crossing
Concern of Veterinary Authorities With Respect to Borders Crossing NURUL HUSNA ZULKIFLI DEPARTMENT OF VETERINARY SERVICES MALAYSIA 19 FEBRUARY 2019 DEPARTMENT OF VETERINARY SERVICES MALAYSIA 01 Overview Under the Ministry of Agriculture and Agro-Based Industries (MOA) Malaysia 02 Responsibility Veterinary competent authority of Malaysia 03 Scope Responsible in performing risk analysis as well as development of import requirements and for issuance of veterinary health certificate (as stated under the Provisions of Animals Act 1953, Section 16. DEPARTMENT OF QUARANTINE & INSPECTION SERVICES MALAYSIA (MAQIS) 01 Overview Under the Ministry of Agriculture and Agro-Based Industries (MOA) Malaysia 02 Responsibility One stop centre for quarantine and inspection services 03 Scope Responsible in the enforcement of written laws at entry point, quarantine stations and quarantine premises as well as in the issuance of permits, licenses and certificates of import and export DVS as a competent authority responsible in the approval of animal and animal products movement STATE DVS IN MALAYSIA 1. Perlis 2. Kedah 3. Pulau Pinang 4. Kelantan 5. Terengganu 6. Perak 7. Pahang 8. Selangor 9. Putrajaya (Headquarters) 10. Negeri Sembilan 11. Melaka 12. Johor 13. Labuan MAQIS as main agency responsible in quarantine, inspection and enforcement STATE MAQIS IN MALAYSIA 1. Selangor/Negeri Sembilan 2. Johor/Melaka 3. Pulau Pinang 4. Kedah 5. Kelantan 6. Perlis 7. Perak 8. Labuan 9. Pahang/Terengganu MAQIS Quarantine Station Labuan KLIA • KLIA Quarantine Station • Padang -

Senarai GM Kelantan
BIL GIATMARA ALAMAT TELEFON & FAKS KURSUS YANG DITAWARKAN Wisma Amani, Lot PT 200 & 201, 09-7422990 (Am) Pejabat GIATMARA Negeri Taman Maju, Jalan Sultan Yahya Petra, 09-7422992 (Faks) 15200 Kota Bharu, Kelantan Darul Naim PENDAWAI ELEKTRIK (PW2) 09-7787311, PENDAWAI ELEKTRIK (PW4 - 36 BULAN) 1 Bachok (4) Lot 665, Kampung Serdang Baru, 16310 Bachok 09-7787312 (F) TEKNOLOGI AUTOMOTIF FASHION AND DRESSMAKING INDUSTRIAL MAINTENANCE 09-9285171, 2 Gua Musang (3) Felda Chiku 5, 18300 Gua Musang TEKNOLOGI MOTOSIKAL 09-9287637 (F) TEKNOLOGI AUTOMOTIF PENDAWAI ELEKTRIK (PW2) 09-9468553, FASHION AND DRESSMAKING 3 Jeli (4) Kampung Rahmat, 17700 Ayer Lanas 09-9468550 (F) TEKNOLOGI AUTOMOTIF TEKNOLOGI BAIKPULIH & MENGECAT KENDERAAN FASHION AND DRESSMAKING HIASAN DALAMAN 09-7880211, 4 Ketereh (5) Lot 236, Kuarters KADA Ketereh, 16450 Ketereh SENI SULAMAN KREATIF 09-7880212 (F) SENI SULAMAN KREATIF (SULAMAN MESIN) SENI SULAMAN KREATIF (SULAMAN TANGAN) PENDAWAI ELEKTRIK (PW2) PENDAWAI ELEKTRIK (PW4 - 12 BULAN) 5 Kota Bharu (4) Jalan Telipot, 15150 Kota Bharu 09-7447058 (P/F) TEKNOLOGI AUTOMOTIF TEKNOLOGI ELEKTRONIK AUDIO VISUAL 09-9362689, TEKNOLOGI MOTOSIKAL 6 Kuala Krai (2) Kampung Jelawang, 18200 Dabong, Kuala Krai 09-9361689 (F) FASHION AND DRESSMAKING Lot 2399 Kg Padang Bongor, Kubang Kerian, 16150 CONFECTIONARY AND BAKERY Kota Bharu 09-7666871, 7 Kubang Kerian (3) FASHION AND DRESSMAKING 09-7666872 (F) SOLEKAN DAN TERAPI KECANTIKAN TEKNOLOGI AUTOMOTIF 09-9750016, TEKNOLOGI ELEKTRONIK AUDIO VISUAL 8 Machang (4) Balai Polis Lama, 18500 Machang 09-9750017 -

Direktori Pensijilan Halal Kelantan
DIREKTORI PENSIJILAN HALAL KELANTAN BIL. NAMA SYARIKAT/PREMIS PRODUK JENAMA TARIKH STATUS PRODUK TAMAT SIJIL 1. 1 Adilah Products Kicap, Sos Tomato, Jenama Adilah Julai 2015 Lot 932, Kg. Pasir Hor, 15100 Kota Bharu, Sos Cili, Sos Pencicah, Kelantan. Cuka dan Perasa Ros. No. Tel : 09-7655932 2. 5 Al-Arzaaq (KEL) Sdn. Bhd. Tumis Segera & Jenama ruz Februari 2015 Lot 505, Kampung Chempaka, Perencah Jalan Panji, 16100 Kota Bharu, Kelantan. No. Tel : 09-7737346 3. 6 ALB Healthy And Beauty Kopi Pracampuran Februari 2015 Lot 3067, Kg. Padang Pak Amat, Oktober 2015 Cherang Rotan, Jln. Pasir Puteh- Kota Bharu, 16800 Pasir Puteh. 097867228 4. 7 Al-Baitif Food Industries (M) Sdn. Bhd. Karipap, Pau Goreng, Jenama Al-Baitif September 2015 PT 2425,No 3,Jln 4/7,Kawasan Perindustrian Samosa, Popia, Kuih Pengkalan Chepa II,16100 Kota Bharu, Kelantan. Bom dan Sardin No. Tel : 09-7868085 Gulung 5. 8 Amal Food Industries Sdn. Bhd. Perencah Segera Jenama Amal Oktober 2015 169, Ayer Lanas, Jeli 17700 Ayer Lanas, Kelantan. Tandoori No. Tel : 09-9308169/Shahrizat 014-5330329 6. Arrazi Marketing Sdn. Bhd. Arrazi Kopi Arrazi Mei 2016 No 621 & 621A, Jln Kubang Panjang, Pracampuran, Arrazi 17000 PAsir Mas Goat Milk Cafe-White 019-6477642 Coffe, Banna Malt Coklat, Cocoa Bestari, Damai Susu Kambing Asli. 7. 1 Ayusri Enterprise Kacang Sira Jenama: Ayu Oktober 2015 0 4371, Jalan Kebun Sultan, Page 1 15300 Kota Bharu, Kelantan 019-4881968, 017-9371978 8. 1 Azam Ternak Sdn. Bhd. Ayam Proses. Februari 2015 1 No.1240-E, Kg. Cherang, Jalan Yaakubiah, 15200 Kota Bharu, Kelantan. -

Measuring Health Clinics' Workload Pressure in Kelantan Using The
Malaysian Journal of Medicine and Health Sciences (eISSN 2636-9346) ORIGINAL ARTICLE Measuring Health Clinics’ Workload Pressure in Kelantan Using the Workload Indicator of Staffing Needs Ahmad Zulfahmi Mohd Kamaruzaman1, Mohd Ismail Ibrahim1, Anees Abdul Hamid2 1 Department of Community Medicine, School of Medical Sciences, Universiti Sains Malaysia 16150 Kota Bharu, Kelantan, Malaysia 2 Kelantan State Health Department, 15200 Kota Bharu, Kelantan, Malaysia ABSTRACT Introduction: Proper distribution of human resources is an important factor ensuring high-quality performance and sustained service quality. The aim of this study was determining the workload pressure among medical officers in health clinics (HCs) in Kelantan. Method: A record review survey was conducted between January and April 2019 using human resources data for 2018 involving HCs in Kelantan. It included all the HCs in Kelantan and excluded community clinics. Workload pressure was determined using a tool known as Workload Indicator of Staffing Needs, developed by World Health Organization. A high workload pressure was defined as a ratio between required and acquired medical officers of less than 1. The data were presented descriptively using as frequencies and percentages. Results: All 85 HCs in Kelantan were involved in the study; 90% (9/10) of the Kelantan districts recorded high work- load pressure. Moreover, 68.2% (58/85) HCs had high workload pressure. Tanah Merah, Tumpat, Pasir Mas, and Kota Bharu had the most HCs with high workload pressure, and most such HCs were found in areas with a high-den- sity population, requiring huge coverage. Conclusion: The Kelantan State Health Department should develop better human resource distribution strategies to ensure the sustainability of quality care in HCs. -

Master of Medicine 2019/2020
PPSP/PG/OPH/CP2/IR1 MASTER OF MEDICINE OPHTHALMOLOGY Universiti Sains Malaysia 2019/2020 Malaysian Universities Conjoint Committee of Ophthalmology MASTER OF MEDICINE (OPHTHALMOLOGY) BY Professor Dr. Shatriah Ismail LIST OF CONTRIBUTORS Associate Professor Datin Dr. Zunaina Embong Professor Dr. Wan Hazabbah Wan Hitam Professor Dr. Liza Sharmini Ahmad Tajudin Dr. Evelyn Tai Li Min 1st Edition - May 2005 2nd Edition - May 2006 3rd Edition - May 2007 4th Edition - May 2008 5th Edition - May 2009 6th Edition - May 2010 7th Edition - May 2011 8th Edition - May 2012 9th Edition - May 2013 10th Edition - May 2014 11th Edition - May 2015 12th Edition - May 2016 13th Edition - May 2017 14th Edition - May 2018 15th Edition - May 2019 Preface Welcome to the Master of Medicine (Ophthalmology) program at Universiti Sains Malaysia (USM). As a prior graduate of this program, it is my privilege to write the preface for this guidebook. As with any course one elects to undertake, one requires at least an understanding of the course structure, the syllabus, and the requirements for each phase, in order to successfully complete the course within a reasonable time. First released in 2005, this guidebook aims to give you an overview of these things and more. As a student, I often referred to my guidebook when in doubt, and even now, still have that original guidebook, although it has undergone many revisions since my time. As goalposts may shift, particularly the pre-requisites for continuous assessment and the examination formats, it would be advisable to refer to a copy of the most recent version of this book. -

KELANTAN P = Parlimen / Parliament N = Dewan Undangan Negeri
KELANTAN P = Parlimen / Parliament N = Dewan Undangan Negeri (DUN) / State Constituencies KAWASAN / STATE PENYANDANG / INCUMBENT PARTI / PARTY P019 TUMPAT KAMAR UDIN BIN JAFFAR PAS N01901 - PENGKALAN KUBOR USTAZ MAT RAZI BN N01902 – KELABORAN MOHAMAD ZAKI BIN IBRAHIM PAS N01903 - PASIR PEKAN AHMAD BIN YAKOB PAS N01904 - WAKAF BHARU CHE ABDULLAH BIN MAT NAWI PAS P020 PENGKALAN CHEPA IZANI BIN HUSIN PAS N02005 – KIJANG WAN UBAIDAH BINTI OMAR PAS N02006 – CHEMPAKA USTAZ FATHAN (AHMAD FATHAN BIN N MAHMOOD @ MAHAMAD) PAS 02007 - PANCHOR MOHD AMAR BIN ABDULLAH PAS P021 KOTA BHARU TAKIYUDDIN BIN HASSAN PAS N02108 - TANJONG MAS ROHANI BINTI IBRAHIM PAS N02109 - KOTA LAMA TAN TENG LOON @ ANUAR TAN B. ABDULLAH PAS N02110 - BUNUT PAYONG RAMLI BIN MAMAT PAS P022 PASIR MAS NIK MOHAMAD ABDUH BIN NIK ABDUL AZIZ PAS N02211 – TENDONG ROZI BIN MUHAMAD PAS N02212 - PENGKALAN PASIR HANIFA BIN AHMAD PAS N02213 - CHETOK ABDUL HALIM BIN ABDUL RAHMAN PAS P023 RANTAU PANJANG SITI ZAILAH BINTI MOHD YUSOFF PAS N02314 – MERANTI MOHD NASSURUDDIN BIN HAJI DAUD PAS N02315 - GUAL PERIOK MOHAMAD BIN AWANG PAS N02316 - BUKIT TUKU ABDUL RASUL BIN MOHAMED PAS P024 KUBANG KERIAN AHMAD BAIHAKI BIN ATIQULLAH PAS N02417 – SALOR HUSAM BIN MUSA PAS N02418 - PASIR TUMBOH ABD RAHMAN BIN YUNUS PAS N02419 - DEMIT MUMTAZ BINTI MD NAWI PAS P025 BACHOK AHMAD MARZUK BIN SHAARY PAS N02520 – TAWANG HASSAN BIN MOHAMOOD PAS N02521 – PERUPOK MOHD HUZAIMY BIN CHE HUSIN PAS N02522 - JELAWAT ABDUL AZZIZ BIN KADIR PAS P026 KETEREH ANNUAR BIN MUSA BN N02623 – MELOR MD. YUSNAN BIN YUSOF PAS N02624 – KADOK AZAMI BIN HJ. MOHD NOR PAS N02625 - KOK LANAS MD. -

Summerville Industrialised Building System (IBS) Siteplan
Introducing A New Construction Method: SummerVille Industrialised Building System (IBS) Siteplan Industrialised building system (IBS) is a term used in Malaysia for a technique of construction where by components are manufactured in a controlled environment, either at site or off site, placed and assembled into construction works. The criteria we aim to achieve from an IBS methodology of building modern homes are: + Safety + Durability PHASE 1 + Economy 216 HOMES + Minimum Maintainence and Quality Assurance + Speed to completion SummerVille PHASE 2 ECRL to Kota Bharu RESIDENCES Advantages of IBS 88 HOMES 11 + IBS is able to utilized unskilled workers in construction, which in regular contruction methods may results in bad quality or defects 25 24 in buildings. It is a smart building system that is quick and requires minimal training. SUMMERVILLE 24 + IBS provides efficiency in the construction process, consequently this will shorten the construction period, which benefits RESIDENCES purchasers who have to pay progressive interest during construction period. 24 27 East Entrance 24 24 + IBS minimises the usage of timber which are not eco-friendly and might result in termite issues in future 12 14 4 West 26 15 Kuantan Entrance 19 22 Exit 17 13 24 24 20 3 16 KotaSAS 2 Access Road IBS Building Materials 5 KOTASAS CENTRAL 13 8 9 23 IBS uses concrete, a strong material which is commonly used in heavy construction that needs high load bearing capacity, such as 18 bridges, high-rise construction, retaining walls, dams, infrastructure works and airports. Rest assured your new home will be of the 21 7 highest quality building materials as well as strong. -

Animals Rules, 1962
Kaedah-Kaedah Binatang 1962 [Kaedah-Kaedah ini pada asalnya telah dibuat dan dicetak dalam versi Bahasa Inggeris sahaja] L.N. 323. ANIMALS ORDINANCE, 1953 IN exercise of the powers conferred by section 86 of the Animals Ordinance, 1953[17/1953], the Minister of Agriculture hereby makes the following Rules: Citation 1. These Rules may be cited as the Animals Rules, 1962. Interpretation 2. In these Rules unless the context otherwise requires- “prescribed landing place” means a place where animals and birds any be imported under the provisions of Rule 3; “State Director” means a Veterinary Officer of a State. Landing places 3. (1) Animals or birds may be imported or exported by land only at- (a) the railway station at Padang Besar; (b) the railway station at Johore Bahru; (c) the Customs Station at Johore Bahru; (d) the Railway Station at Rantau Panjang; (e) the Customs Station at Rantau Panjang; (f) the Animal Quarantine Station at Rantau Panjang; (g) the Customs Station at Bukit Kayu Hitam; (h) the Customs Station at Padang Besar; (i) the Immigration Station at Bukit Bunga; (j) the Customs Station at Pengkalan Hulu; or (k) the Kompleks Sultan Abu Bakar at Tanjung Kupang. 1 (2) Animals or birds may be imported or exported by sea only at- (a) the port of Penang or Butterworth; (b) the port of Port Swettenham, Klang; (c) the Port of Pasir Gudang; (d) the Port of Kuantan; (e) the Customs Station at Pengkalan Kubur; (f) the Port of West Port, Klang; (g) the Port of Lumut; (h) the Port of Tanjung Pelepas; or (i) the Port of Labuan. -

Land Allocation Approach for the Non-Registered Proprietor Flood Victims in Kuala Krai
Land Allocation Approach for the Non-Registered Proprietor Flood Victims in Kuala Krai Mohamad Haizam Mohamed Saraf, Thuraiya Mohd and Siti Fairuz Che Pin To Link this Article: http://dx.doi.org/10.6007/IJARBSS/v8-i1/4068 DOI: 10.6007/IJARBSS/v8-i1/4068 Received: 02 Dec 2017, Revised: 07 Jan 2018, Accepted: 16 Jan 2018 Published Online: 18 Jan 2018 In-Text Citation: (Saraf, Mohd, & Pin, 2018) To Cite this Article: Saraf, M. H. M., Mohd, T., & Pin, S. F. C. (2018). Land Allocation Approach for the Non- Registered Proprietor Flood Victims in Kuala Krai. International Journal of Academic Research in Business and Social Sciences, 8(1), 940–956. Copyright: © 2018 The Author(s) Published by Human Resource Management Academic Research Society (www.hrmars.com) This article is published under the Creative Commons Attribution (CC BY 4.0) license. Anyone may reproduce, distribute, translate and create derivative works of this article (for both commercial and non-commercial purposes), subject to full attribution to the original publication and authors. The full terms of this license may be seen at: http://creativecommons.org/licences/by/4.0/legalcode Vol. 8, No.1, January 2018, Pg. 940 - 956 http://hrmars.com/index.php/pages/detail/IJARBSS JOURNAL HOMEPAGE Full Terms & Conditions of access and use can be found at http://hrmars.com/index.php/pages/detail/publication-ethics International Journal of Academic Research in Business and Social Sciences Vol. 8 , No.1, January 2018, E-ISSN: 2222-6990 © 2018 HRMARS Land Allocation Approach for the Non-Registered Proprietor Flood Victims in Kuala Krai Mohamad Haizam Mohamed Saraf, Thuraiya Mohd and Siti Fairuz Che Pin Faculty of Architecture, Planning and Surveying, Universiti Teknologi MARA, Perak Branch, Seri Iskandar Campus, 32610 Bandar Seri Iskandar, Perak, Malaysia Abstract The purpose of this research is to provide land allocation approach for the non registered proprieotor flood victims in Kuala Krai, Malaysia.