Paper Format
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Central-Karachi
Central-Karachi 475 476 477 478 479 480 Travelling Stationary Inclass Co- Library Allowance (School Sub Total Furniture S.No District Teshil Union Council School ID School Name Level Gender Material and Curricular Sport Total Budget Laboratory (School Specific (80% Other) 20% supplies Activities Specific Budget) 1 Central Karachi New Karachi Town 1-Kalyana 408130186 GBELS - Elementary Elementary Boys 20,253 4,051 16,202 4,051 4,051 16,202 64,808 16,202 81,010 2 Central Karachi New Karachi Town 4-Ghodhra 408130163 GBLSS - 11-G NEW KARACHI Middle Boys 24,147 4,829 19,318 4,829 4,829 19,318 77,271 19,318 96,589 3 Central Karachi New Karachi Town 4-Ghodhra 408130167 GBLSS - MEHDI Middle Boys 11,758 2,352 9,406 2,352 2,352 9,406 37,625 9,406 47,031 4 Central Karachi New Karachi Town 4-Ghodhra 408130176 GBELS - MATHODIST Elementary Boys 20,492 4,098 12,295 8,197 4,098 16,394 65,576 16,394 81,970 5 Central Karachi New Karachi Town 6-Hakim Ahsan 408130205 GBELS - PIXY DALE 2 Registred as a Seconda Elementary Girls 61,338 12,268 49,070 12,268 12,268 49,070 196,281 49,070 245,351 6 Central Karachi New Karachi Town 9-Khameeso Goth 408130174 GBLSS - KHAMISO GOTH Middle Mixed 6,962 1,392 5,569 1,392 1,392 5,569 22,278 5,569 27,847 7 Central Karachi New Karachi Town 10-Mustafa Colony 408130160 GBLSS - FARZANA Middle Boys 11,678 2,336 9,342 2,336 2,336 9,342 37,369 9,342 46,711 8 Central Karachi New Karachi Town 10-Mustafa Colony 408130166 GBLSS - 5/J Middle Boys 28,064 5,613 16,838 11,226 5,613 22,451 89,804 22,451 112,256 9 Central Karachi New Karachi -

Fying Clubs in Pakistan
1 NAME: Tanveer Raza ID: 13005001067 Supervisor: Mr. Kalim Ur Rehman Department: BS Aviation Management School: Institute of Aviation Studies 2 ABBREVIATIONS: ........................................................................................................ 22 CHAPTER 1: EXECUTIVE SUMMARY: ......................................................................................... 23 INTRODUCTION: ......................................................................................................... 25 BACKGROUND: ............................................................................................................ 26 PAKISTAN GENERAL AVIATION LIST: (PCAA) ................................................... 27 CHAPTER 2: PIA FLYING ACADEMY: (PIA) .............................................................................. 29 FLEETS: ........................................................................................................................ 29 Cessna 172: ...................................................................................................................................................... 29 Cessna 152: ...................................................................................................................................................... 30 ACADEMY COURSES: ............................................................................................ 30 Private pilot license (PPL): ........................................................................................................................... -

ESMP-KNIP-Saddar
Directorate of Urban Policy & Strategic Planning, Planning & Development Department, Government of Sindh Educational and Cultural Zone (Priority Phase – I) Subproject Karachi Neighborhood Improvement Project (P161980) Environmental and Social Management Plan (ESMP) October 2017 Environmental and Social Management Plan Final Report Executive Summary Government of Sindh with the support of World Bank is planning to implement “Karachi Neighborhood Improvement Project” (hereinafter referred to as KNIP). This project aims to enhance public spaces in targeted neighborhoods of Karachi, and improve the city’s capacity to provide selected administrative services. Under KNIP, the Priority Phase – I subproject is Educational and Cultural Zone (hereinafter referred to as “Subproject”). The objective of this subproject is to improve mobility and quality of life for local residents and provide quality public spaces to meet citizen’s needs. The Educational and Cultural Zone (Priority Phase – I) Subproject ESMP Report is being submitted to Directorate of Urban Policy & Strategic Planning, Planning & Development Department, Government of Sindh in fulfillment of the conditions of deliverables as stated in the TORs. Overview the Sub-project Educational and Cultural Zone (Priority Phase – I) Subproject forms a triangle bound by three major roads i.e. Strachan Road, Dr. Ziauddin Ahmed Road and M.R. Kayani Road. Total length of subproject roads is estimated as 2.5 km which also forms subproject boundary. ES1: Educational and Cultural Zone (Priority Phase – I) Subproject The following interventions are proposed in the subproject area: three major roads will be rehabilitated and repaved and two of them (Strachan and Dr Ziauddin Road) will be made one way with carriageway width of 36ft. -

The Land of Five Rivers and Sindh by David Ross
THE LAND OFOFOF THE FIVE RIVERS AND SINDH. BY DAVID ROSS, C.I.E., F.R.G.S. London 1883 Reproduced by: Sani Hussain Panhwar The land of the five rivers and Sindh; Copyright © www.panhwar.com 1 TO HIS EXCELLENCY THE MOST HONORABLE GEORGE FREDERICK SAMUEL MARQUIS OF RIPON, K.G., P.C., G.M.S.I., G.M.I.E., VICEROY AND GOVERNOR-GENERAL OF INDIA, THESE SKETCHES OF THE PUNJAB AND SINDH ARE With His Excellency’s Most Gracious Permission DEDICATED. The land of the five rivers and Sindh; Copyright © www.panhwar.com 2 PREFACE. My object in publishing these “Sketches” is to furnish travelers passing through Sindh and the Punjab with a short historical and descriptive account of the country and places of interest between Karachi, Multan, Lahore, Peshawar, and Delhi. I mainly confine my remarks to the more prominent cities and towns adjoining the railway system. Objects of antiquarian interest and the principal arts and manufactures in the different localities are briefly noticed. I have alluded to the independent adjoining States, and I have added outlines of the routes to Kashmir, the various hill sanitaria, and of the marches which may be made in the interior of the Western Himalayas. In order to give a distinct and definite idea as to the situation of the different localities mentioned, their position with reference to the various railway stations is given as far as possible. The names of the railway stations and principal places described head each article or paragraph, and in the margin are shown the minor places or objects of interest in the vicinity. -
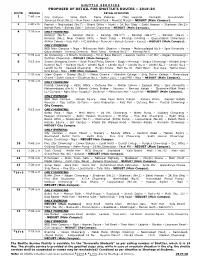
Shuttle Route
SHUTTLE SERVICES PROPOSED OF DETAIL FOR SHUTTLE’S ROUTES – 2019-20 ROUTE TIMINGS DETAIL OF ROUTES 1 7:40 a.m City Campus – Jama Cloth – Radio Pakistan – 7Day Hospital – Numaish – Gurumandir – Jamshed Road (No.3) – New Town – Askari Park – Mumtaz Manzil – NEDUET (Main Campus). 2 7:40 a.m Paposh – Nazimabad (No.7) – Board Office – Hydri – 2K Bus Stop – Sakhi Hassan – Shadman (No.2)– Namak Bank – Sohrab Goth – Gulshan Chowrangi – NEDUET (Main Campus). 4 7:20 a.m ONLY MORNING: Korangi (No.5) – Korangi (No.4) – Korangi (No.31/2) – Korangi (No.21/2) – Korangi (No.2) – Korangi (No.1, Near Chakra Goth) – Nasir Jump – Korangi Crossing – Qayyumabad Chowrangi – Akhtar Colony – Kala Pull – FTC Building – Nursery – Baloch Colony – Karsaz – NEDUET (Main Campus). ONLY EVENING: NED Main Campus – Nipa – Millennium Mall– Stadium – Karsaz – Mehmoodabad No.6 – Iqra University – Qayyumabad – Korangi Crossing – Nasir Jump – Korangi No.21/2 – Korangi No.5. 5 7:45 a.m 4K Chowrangi – 2 Minute Chowrangi – 5C-4 (Bara Market) – Saleem Centre – U.P Mor – Nagan Chowrangi – Gulshan Chowrangi – NEDUET (Main Campus). 6 7:15 a.m Shama Shopping Centre – Shah Faisal Police Station – Bagh-e-Korangi – Singer Chowrangi – Khaddi Stop – Korangi No.5 – Korangi No.6 – Landhi No.6 – Landhi No.5 – Landhi No.4 – Landhi No.3 – Landhi No.1 – Landhi No.89 – Dawood Chowrangi – Murghi Khana – Malir No.15 – Malir Hault – Star Gate – Natha Khan – Drig Road – Nipa – NED Main Campus. 7 7:35 a.m Islam Chowk – Orangi (No.5) – Metro Cinema – Abdullah College – Ship Owner College – Qalandarya Chowk – Sakhi Hassan – Shadman No.1 – Buffer Zone – Fazal Mill – Nipa – NEDUET (Main Campus). -

List of Branches Authorized for Overnight Clearing (Annexure - II) Branch Sr
List of Branches Authorized for Overnight Clearing (Annexure - II) Branch Sr. # Branch Name City Name Branch Address Code Show Room No. 1, Business & Finance Centre, Plot No. 7/3, Sheet No. S.R. 1, Serai 1 0001 Karachi Main Branch Karachi Quarters, I.I. Chundrigar Road, Karachi 2 0002 Jodia Bazar Karachi Karachi Jodia Bazar, Waqar Centre, Rambharti Street, Karachi 3 0003 Zaibunnisa Street Karachi Karachi Zaibunnisa Street, Near Singer Show Room, Karachi 4 0004 Saddar Karachi Karachi Near English Boot House, Main Zaib un Nisa Street, Saddar, Karachi 5 0005 S.I.T.E. Karachi Karachi Shop No. 48-50, SITE Area, Karachi 6 0006 Timber Market Karachi Karachi Timber Market, Siddique Wahab Road, Old Haji Camp, Karachi 7 0007 New Challi Karachi Karachi Rehmani Chamber, New Challi, Altaf Hussain Road, Karachi 8 0008 Plaza Quarters Karachi Karachi 1-Rehman Court, Greigh Street, Plaza Quarters, Karachi 9 0009 New Naham Road Karachi Karachi B.R. 641, New Naham Road, Karachi 10 0010 Pakistan Chowk Karachi Karachi Pakistan Chowk, Dr. Ziauddin Ahmed Road, Karachi 11 0011 Mithadar Karachi Karachi Sarafa Bazar, Mithadar, Karachi Shop No. G-3, Ground Floor, Plot No. RB-3/1-CIII-A-18, Shiveram Bhatia Building, 12 0013 Burns Road Karachi Karachi Opposite Fresco Chowk, Rambagh Quarters, Karachi 13 0014 Tariq Road Karachi Karachi 124-P, Block-2, P.E.C.H.S. Tariq Road, Karachi 14 0015 North Napier Road Karachi Karachi 34-C, Kassam Chamber's, North Napier Road, Karachi 15 0016 Eid Gah Karachi Karachi Eid Gah, Opp. Khaliq Dina Hall, M.A. -

Chapter 4 Environmental Management Consultants Ref: Y8LGOEIAPD ESIA of LNG Terminal, Jetty & Extraction Facility - Pakistan Gasport Limited
ESIA of LNG Terminal, Jetty & Extraction Facility - Pakistan Gasport Limited 4 ENVIRONMENTAL BASELINE OF THE AREA Baseline data being presented here pertain to the data collected from various studies along the physical, biological and socio-economic environment coast show the influence of NE and SW monsoon of the area where the proposed LNG Jetty and land winds. A general summary of meteorological and based terminal will be located, constructed and hydrological data is presented in following operated. Proposed location of project lies within the section to describe the coastal hydrodynamics of boundaries of Port Qasim Authority and very near the area under study. the Korangi Fish Harbour. Information available from electronic/printed literature relevant to A- Temperature & Humidity baseline of the area, surrounding creek system, Port Qasim as well as for Karachi was collected at the The air temperature of Karachi region is outset and reviewed subsequently. This was invariably moderate due to presence of sea. followed by surveys conducted by experts to Climate data generated by the meteorological investigate and describe the existing socio-economic station at Karachi Air Port represents climatic status, and physical scenario comprising conditions for the region. The temperature hydrological, geographical, geological, ecological records for five years (2001-2005) of Karachi city and other ambient environmental conditions of the are being presented to describe the weather area. In order to assess impacts on air quality, conditions. Table 4.1 shows the maximum ambient air quality monitoring was conducted temperatures recorded during the last 5 years in through expertise provided by SUPARCO. The Karachi. baseline being presented in this section is the extract of literature review, analyses of various samples, Summer is usually hot and humid with some surveys and monitoring. -

Public Notice Auction of Gold Ornament & Valuables
PUBLIC NOTICE AUCTION OF GOLD ORNAMENT & VALUABLES Finance facilities were extended by JS Bank Limited to its customers mentioned below against the security of deposit and pledge of Gold ornaments/valuables. The customers have neglected and failed to repay the finances extended to them by JS Bank Limited along with the mark-up thereon. The current outstanding liability of such customers is mentioned below. Notice is hereby given to the under mentioned customers that if payment of the entire outstanding amount of finance along with mark-up is not made by them to JS Bank Limited within 15 days of the publication of this notice, JS Bank Limited shall auction the Gold ornaments/valuables after issuing public notice regarding the date and time of the public auction and the proceeds realized from such auction shall be applied towards the outstanding amount due and payable by the customers to JS Bank Limited. No further public notice shall be issued to call upon the customers to make payment of the outstanding amounts due and payable to JS Bank as mentioned hereunder: Customer Sr. No. Customer's Name Address Balance as on 12th October 2020 Number 1 1038553 ZAHID HUSSAIN MUHALLA MASANDPURSHI KARPUR SHIKARPUR 327,924 2 1012051 ZEESHAN ALI HYDERI MUHALLA SHIKA RPUR SHIKARPUR PK SHIKARPUR 337,187 3 1008854 NANIK RAM VILLAGE JARWAR PSOT OFFICE JARWAR GHOTKI 65110 PAK SITAN GHOTKI 565,953 4 999474 DARYA KHAN THENDA PO HABIB KOT TALUKA LAKHI DISTRICT SHIKARPU R 781000 SHIKARPUR PAKISTAN SHIKARPUR 298,074 5 352105 ABDUL JABBAR FAZALEELAHI ESTATE S HOP -

Rapid Need Assessment Report Monsoon Rains Karachi Division Th Th 24 – 27 August 2020
Rapid Need Assessment Report Monsoon Rains Karachi Division th th 24 – 27 August 2020 Prepared by: Health And Nutrition Development Society (HANDS) Address: Plot #158, Off M9 (Karachi – Hyderabad) Motorway, Gadap Road, Karachi, Pakistan Web: www.hands.org.pk Email: [email protected] Ph: (0092-21) 32120400-9 , +92-3461117771 1 | P a g e Table of Contents 1. Introduction .................................................................................................................... 3 1.1. Background............................................................................................................. 3 1.2. Objectives ............................................................................................................... 3 2. Methodology .................................................................................................................. 4 Situation at Model Town after Heavy Rains ....................................................................... 4 Situation at Model Town after Heavy Rains ....................................................................... 4 3. Findings ......................................................................................................................... 5 3.1. District East............................................................................................................. 6 3.2. Major disaterous events in East district ................................................................... 6 3.3. District Malir ........................................................................................................... -

HSD Retail Price (R/L) a One Filling Station GT Road
TOTAL PARCO PAKISTAN LTD Prices Effective From 01.08.2019 MS Retail Price HSD Retail Price Site Name Address City / Location (R/L) (R/L) A One Filling Station GT Road (Near Zinco Cinema), Gjuranwala. Gujranwala 118.40 133.00 5-A Maripur Road Karachi,Opposite Wazir Mansion Railway station. Karachi A & Y Filling Station Karachi 118.30 133.00 A.B Petroleum Mauza Shera Kot, Bund Road, Lahore Lahore 118.30 133.00 Akbar Filling Station pasroor road, near Bismillah chowk,Sialkot Sialkot 118.70 133.30 Al-Aziz Filling Station Gujranwala - Hafizabad road Qila Didar Singh. Qila Didar Singh 118.40 133.00 Al-Khalil Filling Station G.T Road, Lalamusa. Lalamusa 118.30 133.00 Al-Khalil II Filling Station Mouza Shah Jehani, Lalamusa, Tehsil Kharian District Gujrat. Lalamusa 118.30 133.00 Al-Maalik Filling Station Mouza Mandhyal Tehsil Ferozwala District Sheikhupura 118.30 133.00 New Al-Rehman Filling Station-COCO Plot# 514,515, Block-15, Sector B-1 Quid-e-Azam Town College Road Lahore Lahore 118.40 133.00 Ashraf Filling Station G.T Road (Near Old GTS Addah), Gujrat. Gujrat 118.30 133.00 Askari Filling Station Mouza Auto Wali Tehsil Shakargarh District Shakargarh 118.80 133.50 Bahoo Filling Station 16 & 16 A, Ali Block, New Garden Town, Lahore Lahore 118.40 133.00 Bhatti Petroleum 64-KM Lahore Road, Mor Khunda, Tehsil Nankana Sahib. Nankana 118.40 133.00 Bismillah Trades 95 - Allama Iqbal Road, Garhi Shahu, Mian Mir Sahib, Tehsil Cantt, Lahore Lahore 118.30 133.00 Chaudhary Filling Station (Near Jamia Maki Mosque) Chuna Bhattian, Shalimar Link Road Lahore. -

List of Shareholder-Dividend Withheld
Ghandhara Industries Limited List of shareholders - Dividend Withheld MEMBER NET SR # SHAREHOLDER'S NAME ADDRESS FOLIO PAR ID PAYABLE 1 00002-000 MISBAHUDDIN FAROOQI D-151 BLOCK-B NORTH NAZIMABADKARACHI. 461 2 00004-000 FARHAT AFZA BEGUM MST. 166 ABU BAKR BLOCK NEW GARDEN TOWN,LAHORE 1,302 3 00005-000 ALLAH RAKHA 135-MUMTAZ STREETGARHI SHAHOO,LAHORE. 3,293 4 00006-000 AKHTAR A. PERVEZ C/O. AMERICAN EXPRESS CO.87 THE MALL, LAHORE. 180 5 00008-000 DOST MUHAMMAD C/O. NATIONAL MOTORS LIMITEDHUB CHAUKI ROAD, S.I.T.E.KARACHI. 46 6 00010-000 JOSEPH F.P. MASCARENHAS MISQUITA GARDEN CATHOLIC COOP.HOUSING SOCIETY LIMITED BLOCK NO.A-3,OFF: RANDLE ROAD KARACHI. 1,544 7 00013-000 JOHN ANTHONY FERNANDES A-6, ANTHONIAN APT. NO. 1,ADAM ROAD,KARACHI. 5,004 8 00014-000 RIAZ UL HAQ HAMID C-103, BLOCK-XI, FEDERAL.B.AREAKARACHI. 69 9 00015-000 SAIED AHMAD SHAIKH C/O.COMMODORE (RETD) M. RAZI AHMED71/II, KHAYABAN-E-BAHRIA, PHASE-VD.H.A. KARACHI-46. 214 10 00016-000 GHULAM QAMAR KURD 292/8, AZIZABAD FEDERAL B. AREA,KARACHI. 129 11 00017-000 MUHAMMAD QAMAR HUSSAIN C/O.NATIONAL MOTORS LTD.HUB CHAUKI ROAD, S.I.T.E.,P.O.BOX-2706, KARACHI. 218 12 00018-000 AZMAT NAWAZISH AZMAT MOTORS LIMITED, SC-43,CHANDNI CHOWK, STADIUM ROAD,KARACHI. 1,585 13 00021-000 MIRZA HUSSAIN KASHANI HOUSE NO.R-1083/16,FEDERAL B. AREA, KARACHI 434 14 00023-000 RAHAT HUSSAIN PLOT NO.R-483, SECTOR 14-B,SHADMAN TOWN NO.2, NORTH KARACHI,KARACHI. -

Environmental and Social Impact Assessment (ESIA) of BQPS-III 900 MW RLNG Based Combined Cycle Power Plant Karachi, Pakistan
K-Electric Limited Environmental & Social Impact Assessment (ESIA) of BQPS-III 900 MW RLNG Based Combined Cycle Power Project (BQPS-III RLNG CCPP). Final Report Environmental and June, 2017 Social Impact Assessment (ESIA) of BQPS-III 900 MW RING Based Combined Cycle Power Plant Global Environmental Management Services (Pvt.) Ltd. Z"“1 Floor, Aiwan-e-Sanat, ST-4/2, Sector 23, Korangi Industrial Area, Karachi (BQPS-III RLNG CCPP). GEMS Ph: (92-21) 35113804-5; Fax: (92-21) 35113806; Email: [email protected] Global Environmental Management Services (Pvt.) Ltd. 2nd Floor, Aiwan-e-Sanat, ST-4/2, Sector 23, Korangi Industrial Area, Karachi GEMS Rh: (92-21) 35113804-5; Fax: (92-21) 35113806; Email; [email protected] Environmental and Social Impact Assessment (ESIA) of BQPS-III 900 MW RLNG Based Combined Cycle Power Plant Karachi, Pakistan ENVIRONMENTAL CONSULTANT'S PROFILE AND INTRODUCTION ES Global Environmental Management Services (Pvt.) Ltd. (GEMS) is an Environmental EXECUTIVE SUMMARY Consultancy which provides broad range of Environmental Solutions which are and not GEMS limited to Environmental Audits, Initial Environmental Examinations (IEE), Environmental and Social Impact Assessments (ESIA), Baseline studies and Training & Capacity building. GEMS is one of the few environmental firm having its own renowned ISO 17025 OVERVIEW Certified Environmental Laboratory by the name of Global Environmental Laboratory (Pvt) Ltd. Study Type BACKGROUND INFORMATION AND NEED ASSESSMENT OF THE PROPOSED Environmental and Social Impact Assessment (ESIA). PROJECT Study Title ESIA of BQPS-III 900 MW RLNG Based Combined Cycle Power Project Pakistan is in the midst of a severe energy crisis that largely stemmed from mismanagement of natural (BQPS-III RING CCPP).