Interbiotech Arecoline Bromide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Muscarinic Acetylcholine Receptor
mAChR Muscarinic acetylcholine receptor mAChRs (muscarinic acetylcholine receptors) are acetylcholine receptors that form G protein-receptor complexes in the cell membranes of certainneurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibersin the parasympathetic nervous system. mAChRs are named as such because they are more sensitive to muscarine than to nicotine. Their counterparts are nicotinic acetylcholine receptors (nAChRs), receptor ion channels that are also important in the autonomic nervous system. Many drugs and other substances (for example pilocarpineand scopolamine) manipulate these two distinct receptors by acting as selective agonists or antagonists. Acetylcholine (ACh) is a neurotransmitter found extensively in the brain and the autonomic ganglia. www.MedChemExpress.com 1 mAChR Inhibitors & Modulators (+)-Cevimeline hydrochloride hemihydrate (-)-Cevimeline hydrochloride hemihydrate Cat. No.: HY-76772A Cat. No.: HY-76772B Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic receptor agonist, is a candidate therapeutic drug for receptor agonist, is a candidate therapeutic drug for xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR The general pharmacol. properties of this drug on the The general pharmacol. properties of this drug on the gastrointestinal, urinary, and reproductive systems and other… gastrointestinal, urinary, and reproductive systems and other… Purity: >98% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 10mM x 1mL in DMSO, Size: 10mM x 1mL in DMSO, 1 mg, 5 mg 1 mg, 5 mg AC260584 Aclidinium Bromide Cat. No.: HY-100336 (LAS 34273; LAS-W 330) Cat. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
BMJ Open: first published as 10.1136/bmjopen-2018-027935 on 5 May 2019. Downloaded from BMJ Open is committed to open peer review. As part of this commitment we make the peer review history of every article we publish publicly available. When an article is published we post the peer reviewers’ comments and the authors’ responses online. We also post the versions of the paper that were used during peer review. These are the versions that the peer review comments apply to. The versions of the paper that follow are the versions that were submitted during the peer review process. They are not the versions of record or the final published versions. They should not be cited or distributed as the published version of this manuscript. BMJ Open is an open access journal and the full, final, typeset and author-corrected version of record of the manuscript is available on our site with no access controls, subscription charges or pay-per-view fees (http://bmjopen.bmj.com). If you have any questions on BMJ Open’s open peer review process please email [email protected] http://bmjopen.bmj.com/ on September 26, 2021 by guest. Protected copyright. BMJ Open BMJ Open: first published as 10.1136/bmjopen-2018-027935 on 5 May 2019. Downloaded from Treatment of stable chronic obstructive pulmonary disease: a protocol for a systematic review and evidence map Journal: BMJ Open ManuscriptFor ID peerbmjopen-2018-027935 review only Article Type: Protocol Date Submitted by the 15-Nov-2018 Author: Complete List of Authors: Dobler, Claudia; Mayo Clinic, Evidence-Based Practice Center, Robert D. -

PL112-Final.Pdf
WHO Drug Information, Vol. 28, No. 4, 2014 Proposed INN: List 112 International Nonproprietary Names for Pharmaceutical Substances (INN) Notice is hereby given that, in accordance with article 3 of the Procedure for the Selection of Recommended International Nonproprietary Names for Pharmaceutical Substances, the names given in the list on the following pages are under consideration by the World Health Organization as Proposed International Nonproprietary Names. The inclusion of a name in the lists of Proposed International Nonproprietary Names does not imply any recommendation of the use of the substance in medicine or pharmacy. Lists of Proposed (1–109) and Recommended (1–70) International Nonproprietary Names can be found in Cumulative List No. 15, 2013 (available in CD-ROM only). The statements indicating action and use are based largely on information supplied by the manufacturer. This information is merely meant to provide an indication of the potential use of new substances at the time they are accorded Proposed International Nonproprietary Names. WHO is not in a position either to uphold these statements or to comment on the efficacy of the action claimed. Because of their provisional nature, these descriptors will neither be revised nor included in the Cumulative Lists of INNs. Dénominations communes internationales des Substances pharmaceutiques (DCI) Il est notifié que, conformément aux dispositions de l'article 3 de la Procédure à suivre en vue du choix de Dénominations communes internationales recommandées pour les Substances pharmaceutiques les dénominations ci-dessous sont mises à l'étude par l'Organisation mondiale de la Santé en tant que dénominations communes internationales proposées. -
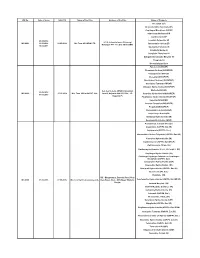
WC No. Date of Issue Valid Till Name of the Firm Address of The
WC No. Date of Issue Valid Till Name of the Firm Address of the Firm Name of Products Irbesartan (EP) Desfesoterodine Succinate (IH) Clopidogrel Bisulphate USP/EP Olmesartan Medoxomil IH Clarithromycin EP 24.05.2016 Losartan Potassium EP Q-1-4, Industrial area ,Ghinrongi WC-0001 13.06.2016 14.05.2019 M/s Teva API INDIA LTD Atorvastatin Calcium EP Malanpur -477 117, Dist -Bhind (MP) 16.02.2017 Quetiapine Fumarate IH Sitagliptin Malate IH Sitagliptin Phosphate IH Dabigatrante Etexilate Mesylate IH Ticagrelor IH Desvenlafaxine Base Famciclovir( IH/USP) Fluvastatin Sodium (IH/USP/EP) Olanzapine(IH/ USP/EP) Irbesartan( IH/USP/EP) Montelukast Sodium(( IH/USP/EP) Quetiapine Fumarate( IH/USP) Diltiazem Hydrochloride(IH/USP/EP) A-2, A-2/1, A-2/2, UPSIDC Industrial Ezetimibe(IH/USP) 22.08.2016 WC-0002 27.05.2019 M/s. Teva API India PVT. Ltd., Area-II, Gajraula-244 235, Dist., J.P. Sertraline Hydrochloride(IH/USP/EP) 07.03.2018 Nagar (U.P) Pioglitazone Hydrochloride(IH/USP/EP) Valsartan(IH/USP/EP) Losartan Potassium(IH/USP/EP) Pregabalin(IH/USP/EP) Atorvastatin Calcium(IH/USP) Caspofungin Acetate(IH) Eletriptan Hydrobromide (IH) Rosuvastatin Calcium (IH/EP) Acamprosate Calcium (Ph. Eur.) Anastrozole (USP/Ph. Eur./IH) Aripiprazole (USP/Ph. Eur.) Atorvastatin Calcium Trihydrate (USP/Ph. Eur./JP) Cinacalcet Hydrochloride (IH) Clarithromycin (USP/Ph. Eur./BP/JP) Clarithromycin Citrate (IH) Clarithromycin Granules 27.5%, 33% & 42% (IH) Clopidogrel Hydrochloride (IH) Clopidogrel Hydrogen Sulphate or Clopidogrel Bisulphate (USP/Ph. Eur.) Colesevelam Hydrochloride (USP) Dapoxetine Hydrochloride (IH) Donepezil Hydrochloride (USP/Ph. Eur./IH) Dutasteride (Ph. -

TOVEDESO® DESFESOTERODINE in SYNDROME OVERACTIVE BLADDER Making the Fesoterodine Profitable
DAR DRUG ASSESSMENT REPORT 04 / 2020 www.dtb.navarra.es @DTB_Navarre TOVEDESO® DESFESOTERODINE IN SYNDROME OVERACTIVE BLADDER Making the fesoterodine profitable IMPORTANT MODEST SOME ADDED NO THERAPEUTIC INSUFFICIENT + THERAPEUTIC THERAPEUTIC VALUE IN SPECIFIC INNOVATION EVIDENCE DATA INNOVATION INNOVATION SITUATIONS SHEET WHAT IS IT? Decreased gastrointestinal motility. Autonomic neuropathy. Urinary antispasmodic. Risk of long QT syndrome and relevant heart diseases (myo- cardial ischemia, arrhythmia, heart failure). INDICATION Symptoms of overactive bladder syndrome. PLACE IN THERAPEUTICS Due to the absence of comparative studies and assimilate POSOLOGY AND METHOD OF ADMINISTRATION data of its prodrug fesoterodine, it is considered desfesotero- Recommended initial dose in adults, including the elderly, dine does not imply an improvement over other urinary an- is 3.5 mg daily. Maximum daily dose 7 mg. Recommended tispasmodics for treatment of overactive bladder syndrome. reevaluating the response after 8 weeks of treatment. Ad- ministration with or without food. PRESENTATIONS Tovedeso® 3.5 mg prolonged-release tablets, EFFECTIVENESS 28 tablets (TEVA PHARMA S.L.U.) € 41.93 Versus placebo. It uses the fesoterodine data, which shows Tovedeso® 7 mg prolonged-release tablets, limited efficacy. Reduction does not reach one over an ave- 28 tablets (TEVA PHARMA S.L.U.) € 67.08 rage of 12 micturitions per 24 hours vs placebo. RISKS Adverse reactions: Antimuscarinic effects: dry mouth, dry Daily cost treatment (€) eye, dyspepsia, constipation, urinary retention. Contraindi- OXYBUTYNIN (15 mg) 0.21 cated: Urinary retention. Gastric retention. Narrow angle glaucoma. Myasthenia gravis. Severe hepatic impairment. TROSPIUM (40 mg) 0.36 Concomitant use with potent CYP3A4 inhibitors. Severe ul- cerative colitis. Toxic megacolon. -

Lääkeaineiden Yleisnimet (INN-Nimet) 31.12.2019
Lääkealan turvallisuus- ja kehittämiskeskus Säkerhets- och utvecklingscentret för läkemedelsområdet Finnish Medicines Agency Lääkeaineiden yleisnimet (INN-nimet) 31.12. -

Muscarinic Acetylcholine Receptor
mAChR Muscarinic acetylcholine receptor mAChRs (muscarinic acetylcholine receptors) are acetylcholine receptors that form G protein-receptor complexes in the cell membranes of certainneurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibersin the parasympathetic nervous system. mAChRs are named as such because they are more sensitive to muscarine than to nicotine. Their counterparts are nicotinic acetylcholine receptors (nAChRs), receptor ion channels that are also important in the autonomic nervous system. Many drugs and other substances (for example pilocarpineand scopolamine) manipulate these two distinct receptors by acting as selective agonists or antagonists. Acetylcholine (ACh) is a neurotransmitter found extensively in the brain and the autonomic ganglia. www.MedChemExpress.com 1 mAChR Antagonists, Agonists, Inhibitors, Modulators & Activators (+)-Cevimeline hydrochloride hemihydrate (-)-Cevimeline hydrochloride hemihydrate ((+)-SNI-2011; (+)-AF102B hydrochloride hemihydrate) Cat. No.: HY-76772A ((-)-SNI-2011; (-)-AF102B hydrochloride hemihydrate) Cat. No.: HY-76772B (+)-Cevimeline hydrochloride hemihydrate (-)-Cevimeline hydrochloride hemihydrate ((+)-SNI-2011), a potent muscarinic receptor ((-)-SNI-2011), a novel muscarinic receptor agonist, is a candidate therapeutic drug for agonist, is a candidate therapeutic drug for xerostomia in Sjogren's syndrome. IC50 value: xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR The general pharmacol. Target: mAChR The general pharmacol. Purity: >98% Purity: >98% Clinical Data: Launched Clinical Data: Launched Size: 10 mM × 1 mL, 1 mg, 5 mg Size: 10 mM × 1 mL, 1 mg, 5 mg (R,R)-Glycopyrrolate ((R,R)-Glycopyrronium bromide; (Rac)-VU 6008667 (R,R)-Glycopyrrolate bromide) Cat. No.: HY-B0761 Cat. No.: HY-101281A (R,R)-Glycopyrrolate ((R,R)-Glycopyrronium (Rac)-VU 6008667 is a selective negative (bromide); (R,R)-Glycopyrrolate (bromide)) is an allosteric modulator of muscarinic acetylcholine anticholinergic agent. -

Literature Search Strategy for Treatment of Stable
Literature search strategy for Treatment of stable chronic obstructive pulmonary disease: a protocol for a systematic review and evidence map Claudia C. Dobler,1 Magdoleen H Farah,1 Allison S. Morrow,1 Mouaz Alsawas,1 Raed Benkhadra1 Bashar Hasan,1 Larry J Prokop,2 Zhen Wang,1 M. Hassan Murad1 1) Evidence-Based Practice Center, Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Rochester, Minnesota, USA 2) Library Public Services, Mayo Clinic, Rochester, Minnesota, USA. Ovid Part 1 Database(s): Embase 1988 to 2018 Week 16, EBM Reviews - Cochrane Database of Systematic Reviews 2005 to April 11, 2018, Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non- Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present Search Strategy: # Searches Results exp Pulmonary Disease, Chronic Obstructive/dh, dt, px, rh, su, th [Diet Therapy, Drug 1 18829 Therapy, Psychology, Rehabilitation, Surgery, Therapy] exp chronic obstructive lung disease/dm, dt, rh, su, th [Disease Management, Drug Therapy, 2 27067 Rehabilitation, Surgery, Therapy] 3 1 or 2 45896 ("chronic airflow disease*" or "chronic airflow disorder*" or "chronic airflow limitation*" or "chronic airflow obstruction*" or "chronic airway disease*" or "chronic airway disorder*" or "chronic airway limitation*" or "chronic airway obstruction*" or "chronic bronchitis" or "chronic obstructive airflow disease*" or "chronic obstructive airflow disorder*" or "chronic obstructive airway disease*" or "chronic obstructive airway disorder*" -

INN-Nimet 1 27.11.2018 a Abacavir Abacavirum Abakaviiri Abagovomab
INN-nimet Lääkealan turvallisuus- ja kehittämiskeskus Säkerhets- och utvecklingscentret för läkemedelsområdet Finnish Medicines Agency 27.11. -

WC No. Date of Issue Valid Till Name of the Firm Address of the Firm Name of Products Irbesartan
WC No. Date of Issue Valid Till Name of the Firm Address of the Firm Name of Products Irbesartan (EP) Desfesoterodine Succinate (IH) Clopidogrel Bisulphate USP/EP Olmesartan Medoxomil IH Clarithromycin EP 24.05.2016 Losartan Potassium EP Q-1-4, Industrial area ,Ghinrongi WC-0001 13.06.2016 14.05.2019 M/s Teva API INDIA LTD Atorvastatin Calcium EP Malanpur -477 117, Dist -Bhind (MP) 16.02.2017 Quetiapine Fumarate IH Sitagliptin Malate IH Sitagliptin Phosphate IH Dabigatrante Etexilate Mesylate IH Ticagrelor IH Desvenlafaxine Base Famciclovir( IH/USP) Fluvastatin Sodium (IH/USP/EP) Olanzapine(IH/ USP/EP) Irbesartan( IH/USP/EP) Montelukast Sodium(( IH/USP/EP) Quetiapine Fumarate( IH/USP) Diltiazem Hydrochloride(IH/USP/EP) A-2, A-2/1, A-2/2, UPSIDC Industrial Ezetimibe(IH/USP) 22.08.2016 WC-0002 27.05.2019 M/s. Teva API India PVT. Ltd., Area-II, Gajraula-244 235, Dist., J.P. Sertraline Hydrochloride(IH/USP/EP) 07.03.2018 Nagar (U.P) Pioglitazone Hydrochloride(IH/USP/EP) Valsartan(IH/USP/EP) Losartan Potassium(IH/USP/EP) Pregabalin(IH/USP/EP) Atorvastatin Calcium(IH/USP) Caspofungin Acetate(IH) Eletriptan Hydrobromide (IH) Rosuvastatin Calcium (IH/EP) Acamprosate Calcium (Ph. Eur.) Anastrozole (USP/Ph. Eur./IH) Aripiprazole (USP/Ph. Eur.) Atorvastatin Calcium Trihydrate (USP/Ph. Eur./JP) Cinacalcet Hydrochloride (IH) Clarithromycin (USP/Ph. Eur./BP/JP) Clarithromycin Citrate (IH) Clarithromycin Granules 27.5%, 33% & 42% (IH) Clopidogrel Hydrochloride (IH) Clopidogrel Hydrogen Sulphate or Clopidogrel Bisulphate (USP/Ph. Eur.) Colesevelam Hydrochloride (USP) Dapoxetine Hydrochloride (IH) Donepezil Hydrochloride (USP/Ph. Eur./IH) Dutasteride (Ph.