Johnson & Johnson
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From the Academy
FROM THE ACADEMY Joint American Academy of DermatologyeNational Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy Craig A. Elmets, MD (Co-Chair),a HenryW.Lim,MD,b Benjamin Stoff, MD, MA,c Cody Connor, MD,a Kelly M. Cordoro, MD,d Mark Lebwohl, MD,e AprilW.Armstrong,MD,MPH,f Dawn M. R. Davis, MD,g Boni E. Elewski, MD,a Joel M. Gelfand, MD, MSCE,h Kenneth B. Gordon, MD,i AliceB.Gottlieb,MD,PhD,j Daniel H. Kaplan, MD, PhD,k Arthur Kavanaugh, MD,l Matthew Kiselica, BA/BS,m Dario Kivelevitch, MD,n Neil J. Korman, MD, PhD,o Daniela Kroshinsky, MD, MPH,p Craig L. Leonardi, MD,q Jason Lichten, MD,m NehalN.Mehta,MD,MSCE,r Amy S. Paller, MD,s Sylvia L. Parra, MD,t Arun L. Pathy, MD,u Elizabeth A. Farley Prater, MD,v Reena N. Rupani, MD,e Michael Siegel, PhD,m BruceE.Strober,MD,PhD,w,x Emily B. Wong, MD,y Jashin J. Wu, MD,z Vidhya Hariharan, PhD,aa and Alan Menter, MD (Co-Chair)n Birmingham, Alabama; Detroit, Michigan; Atlanta, Georgia; San Francisco, Los Angeles, San Diego, and Irvine, California; New York, New York; Rochester, Minnesota; Philadelphia and Pittsburgh, Pennsylva- nia; Milwaukee, Wisconsin; Portland, Oregon; Dallas and San Antonio, Texas; Cleveland, Ohio; Boston, Massachusetts; St. Louis, Missouri; Bethesda, Maryland; Chicago and Rosemont, Illinois; Sumter, South Carolina; Centennial, Colorado; Oklahoma City, Oklahoma; Farmington, Connecticut; and Waterloo, Ontario, Canada Psoriasis is a chronic inflammatory disease involving multiple organ systems and affecting approximately 3.2% of the world’s population. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

Johnson & Johnson
JOHNSON & JOHNSON FORM 10-K (Annual Report) Filed 02/22/13 for the Period Ending 12/30/12 Address ONE JOHNSON & JOHNSON PLZ NEW BRUNSWICK, NJ 08933 Telephone 732-524-2455 CIK 0000200406 Symbol JNJ SIC Code 2834 - Pharmaceutical Preparations Industry Biotechnology & Drugs Sector Healthcare Fiscal Year 12/12 http://www.edgar-online.com © Copyright 2013, EDGAR Online, Inc. All Rights Reserved. Distribution and use of this document restricted under EDGAR Online, Inc. Terms of Use. UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 30, 2012 Commission file number 1-3215 JOHNSON & JOHNSON (Exact name of registrant as specified in its charter) New Jersey 22-1024240 (State of incorporation) (I.R.S. Employer Identification No.) One Johnson & Johnson Plaza New Brunswick, New Jersey 08933 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (732) 524-0400 SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT Title of each class Name of each exchange on which registered Common Stock, Par Value $1.00 New York Stock Exchange Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Annual Report on Annual Reports 2016
ANNUAL REPORT ON ANNUAL REPORTS 2016 TOP 400 ANNUAL REPORTS WHO RANKS WHERE? 100 ANNUALS IN BRIEF BEST REPORTING PRACTICES Company Value > Report Value Annual Report on Annual Reports 2016 Contents Report rating scale 3 Top 400 annual reports 4 Who ranks where? 25 From ABB to ZTE 100 annuals in brief 58 From Abbott to Yamaha How important is the annual report today? 92 Views from Cecilia Ketels, Kellie Friery, Renee Carter, David Robinson, Kaevan Gazdar, Elena Moskvina, Thomas Rosenmayr, Rob Stangroom, Andrey Kozhevnikov, Ana Santamarina, Katie Holcomb, Ananda Jagoda Best practices on key report attibutes 100 Strategy, message, investor information, risks, style, online… How we make it 121 How is your report doing? The report scan 127 The report rating panel 128 Robert Berick, Susan Blesener, Renee Carter, Vero Escarmelle, Helena Fournial, Kaevan Gazdar, Mike Guillaume, Pradip Seth, Eva Wolosiuk Making reports pay off 133 e.com – ReportWatch 135 2 Report rating scale A+ ééééé First-rate A éééé(é) Excellent A- éééé Very good B+ ééé(é) Sound B ééé Average B- éé(é) Uneven C+ éé Common C é(é) Substandard C- é Poor D (é) Uncompetitive 3 Top 400 annual reports AkzoNobel (No. 1) Electrolux (No. 2 ) SCA (No. 3) Volvo (No. 4) 4 Report rank Company Country Report rating Compare 1 AKZONOBEL Netherlands A+ DUPONT 2 ELECTROLUX Sweden A+ WHIRLPOOL 3 SCA Sweden A+ KIMBERLY-CLARK 4 VOLVO Sweden A+ DAIMLER 5 POTASHCORP Canada A+ AGRIUM 6 ATLAS COPCO Sweden A SANDVIK 7 STORA ENSO Finland A UPM 8 BOLIDEN Sweden A GLENCORE 9 WIENERBERGER Austria A BORAL 10 -

Manufacturers and Wholesalers Street
Nevada AB128 Code of Conduct Compliant Companies Manufacturers and Wholesalers Street City ST Zip 10 Edison Street LLC 13 Edison Street LLC Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Electrophysiology (including Kalila Medical 2- 2016)) Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Medical Optics Abbott Molecular Division Abbott Nutrition Products Division Abbott Vascular Division (includes Tendyne 9-2015) AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 Acadia Phamaceuticals 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Accelero Health Partners, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Accuri Cyometers, Inc. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10532 AcriVet, Inc. Actavis W.C. Holding, Inc. Morris Corporate Center III, 400 Interpace Parkway Parsippany NJ 07054 Actavis , Inc. Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Activis 400 Interpace parkway Parsippany NJ 07054 A-Dec, Inc. 2601 Crestview Dr. Newberg OR 97132 Advanced Respiratory, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Advanced Vision Research, Inc., dba Akorn Consumer Health Aegerion Pharmaceuticals, Inc. 101 Main Street, Suite 1850 Cambridge MA 02142 Aesculap Implant Systems, Inc. Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesthera Corporation Afaxys, Inc. PO Box 20158 Charleston SC 29413 AGMS, Inc. Akorn (New Jersey) Inc. Page 1 of 23 Pages 2/15/2017 Nevada AB128 Code of Conduct Compliant Companies Akorn AG (formerly Excelvision AG) Akorn Animal Health, Inc. -

Towards the Creation of Polymer Composites Which Can Be
TOWARDS THE CREATION OF POLYMER COMPOSITES WHICH CAN BE REFILLED WITH ANTIBIOTICS AFTER IMPLANTATION FOR INFECTION TREATMENT By ERIKA LEAH CYPHERT Submitted in partial fulfillment of the requirements For the degree of Doctor of Philosophy Dissertation Advisor: Horst von Recum, Ph.D. Department of Biomedical Engineering CASE WESTERN RESERVE UNIVERSITY January 2021 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of Erika Leah Cyphert Candidate for the Doctor of Philosophy degree*. (signed) Steven Eppell, Ph.D. (chair of committee) Horst von Recum, Ph.D. Eben Alsberg, Ph.D. Agata Exner, Ph.D. Jonathan Pokorski, Ph.D. (date) September 25, 2020 *We also certify that written approval has been obtained for any proprietary material contained therein. 2 To my grandparents with love – Phil and Ann Cyphert Ted and Dorothy Lippold Florence Miller 3 TABLE OF CONTENTS TABLE OF CONTENTS………………………………………………………………..4 LIST OF TABLES…………………………………………………………………..….10 LIST OF FIGURES…………………………………………………………………….13 LIST OF ABBREVIATIONS……………………………………………………….…21 ACKNOWLEDGEMENTS……………………………………………………………24 ABSTRACT…………………………………………………………………………..…27 CHAPTER 1: DIAGNOSIS AND BIOMATERIAL-BASED TREATMENTS FOR PERIPROSTHETIC JOINT INFECTIONS………………………………………….29 1.1. LIMITATIONS OF CLINICAL TREATMENT OF PERIPROSTHETIC INFECTION…………………………………………………………………30 1.1.1. INTRODUCTION…………………………………………….…30 1.1.2. ISOLATION OF MICROBIAL ORGANISMS………………....33 1.1.3. POSSIBLE UNDERLYING PATIENT COMORBIDITIES……33 1.1.4. BIOFILM FORMATION AND BACTERIAL RESISTANCE…34 4 1.1.5. REVISION PROCEDURES/INITIAL TREATMENT FAILURES....................................................................................36 1.1.6. SUMMARY……………………………………………………...37 1.2. NOVEL TREATMENT MODALITIES FOR PJIS………………………...38 1.2.1. LIMITATIONS WITH TRADITIONAL ANTIBIOTIC-LADEN PMMA BONE CEMENT……………………………………..…38 1.2.2. COMMERCIALLY AVAILABLE ALTERNATIVE BIOMATERIALS FOR ANTIBIOTIC-LADEN PMMA BONE CEMENT………………………………………………………...40 1.2.3. -
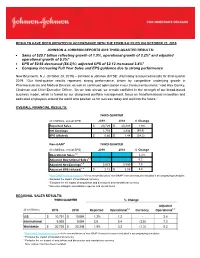
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures -

FDA Listing of Authorized Generics As of July 1, 2021
FDA Listing of Authorized Generics as of July 1, 2021 Note: This list of authorized generic drugs (AGs) was created from a manual review of FDA’s database of annual reports submitted to the FDA since January 1, 1999 by sponsors of new drug applications (NDAs). Because the annual reports seldom indicate the date an AG initially entered the market, the column headed “Date Authorized Generic Entered Market” reflects the period covered by the annual report in which the AG was first reported. Subsequent marketing dates by the same firm or other firms are not included in this listing. As noted, in many cases FDA does not have information on whether the AG is still marketed and, if not still marketed, the date marketing ceased. Although attempts have been made to ensure that this list is as accurate as possible, given the volume of d ata reviewed and the possibility of database limitations or errors, users of this list are cautioned to independently verify the information on the list. We welcome comments on and suggested changes (e.g., additions and deletions) to the list, but the list may include only information that is included in an annual report. Please send suggested changes to the list, along with any supporting documentation to: [email protected] A B C D E F G H I J K L M N O P Q R S T U V X Y Z NDA Applicant Date Authorized Generic Proprietary Name Dosage Form Strength Name Entered the Market 1 ACANYA Gel 1.2% / 2.5% Bausch Health 07/2018 Americas, Inc. -

Annual Report
ANNUAL REPORT 2019 MARCH 2020 To Our Shareholders Alex Gorsky Chairman and Chief Executive Officer By just about every measure, Johnson & These are some of the many financial and Johnson’s 133rd year was extraordinary. strategic achievements that were made possible by the commitment of our more than • We delivered strong operational revenue and 132,000 Johnson & Johnson colleagues, who adjusted operational earnings growth* that passionately lead the way in improving the health exceeded the financial performance goals we and well-being of people around the world. set for the Company at the start of 2019. • We again made record investments in research and development (R&D)—more than $11 billion across our Pharmaceutical, Medical Devices Propelled by our people, products, and and Consumer businesses—as we maintained a purpose, we look forward to the future relentless pursuit of innovation to develop vital with great confidence and optimism scientific breakthroughs. as we remain committed to leading • We proudly launched new transformational across the spectrum of healthcare. medicines for untreated and treatment-resistant diseases, while gaining approvals for new uses of many of our medicines already in the market. Through proactive leadership across our enterprise, we navigated a constant surge • We deployed approximately $7 billion, of unique and complex challenges, spanning primarily in transactions that fortify our dynamic global issues, shifting political commitment to digital surgery for a more climates, industry and competitive headwinds, personalized and elevated standard of and an ongoing litigious environment. healthcare, and that enhance our position in consumer skin health. As we have experienced for 133 years, we • And our teams around the world continued can be sure that 2020 will present a new set of working to address pressing public health opportunities and challenges. -

Johnson & Johnson Annonce Que L'exécution De L'offre Publique D
Press Contacts: Amy Jo Meyer Johnson & Johnson annonce que l'Exécution de l'Offre Publique +1 (732) 524 6678 d'Acquisition visant Actelion est prévue pour le 16 juin 2017 [email protected] Annonce avoir reçu toutes les autorisations réglementaires nécessaires à Ernie Knewitz +1 (917) 697-2318 l'exécution de l'acquisition d'Actelion [email protected] Investor Contacts: NEW BRUNSWICK, N.J. – 9 juin 2017 – Johnson & Johnson (NYSE:JNJ) a Joseph J. Wolk annoncé aujourd'hui qu'avec la réception, ce jour, de l'approbation de l'acquisition +1 (732) 524-1142 projetée visant Actelion Ltd (SIX : ATLN) par la Commission européenne (CE), toutes les autorisations réglementaires nécessaires à l'exécution de la transaction Lesley Fishman +1 (732) 524-3922 ont été reçues. Johnson & Johnson s'attend à ce que l'exécution de l'offre publique d'acquisition entièrement en espèces de sa filiale suisse Janssen Holding GmbH, portant sur toutes les actions en mains du public d'Actelion Ltd au prix de $280 par action, payable en dollars américains, ait lieu le 16 juin 2017. Comme annoncé précédemment, dans le cadre de la transaction, Actelion transférera ses activités en matière de recherche médicamenteuse et ses actifs relatifs au développement préclinique dans une société biopharmaceutique suisse nouvellement constituée ("Idorsia Ltd"). Il est prévu que les actions d'Idorsia Ltd soient distribuées aux actionnaires d'Actelion au moyen d'un dividende en nature et qu'elles soient cotées à la SIX Swiss Exchange le jour de l'exécution de l'offre publique d'acquisition. Une filiale de Johnson & Johnson détiendra initialement 9.9 pour cent des actions d'Idorsia Ltd et elle dispose du droit d'éventuellement augmenter sa participation jusqu'à concurrence de 32 pour cent au moyen d'une obligation convertible. -

HIGHLIGHTS of PRESCRIBING INFORMATION TRACLEER® (Bosentan) Tablets These Highlights Do Not Include All the Information Needed to Use TRACLEER Safely and Effectively
HIGHLIGHTS OF PRESCRIBING INFORMATION TRACLEER® (bosentan) tablets These highlights do not include all the information needed to use TRACLEER safely and effectively. See full prescribing information for TRACLEER. • Reduce the dose and closely monitor patients developing aminotransferase TRACLEER® (bosentan) tablets, for oral use elevations more than 3 X Upper Limit of Normal (ULN) (2.1). ® TRACLEER (bosentan) tablets for oral suspension --------------------------- DOSAGE FORMS AND STRENGTHS --------------------------- Initial U.S. Approval: 2001 • Film-coated tablet: 62.5 mg and 125 mg (3) WARNING: RISKS OF HEPATOTOXICITY and EMBRYO-FETAL TOXICITY • Tablet for oral suspension: 32 mg (3) See full prescribing information for complete boxed warning. ----------------------------------- CONTRAINDICATIONS ----------------------------------- TRACLEER is available only through a restricted distribution program called • Pregnancy (4.1) the Bosentan REMS Program because of these risks (5.3): • Use with Cyclosporine A (4.2) Elevations of liver aminotransferases (ALT, AST) and liver failure have been • Use with Glyburide (4.3) reported with TRACLEER (5.1). • Hypersensitivity (4.4) • Measure liver aminotransferases prior to initiation of treatment and then -----------------------------WARNINGS AND PRECAUTIONS ----------------------------- monthly (2.1, 5.1). • Fluid retention: May require intervention (5.4). • Discontinue TRACLEER if aminotransferase elevations are accompanied by • Pulmonary veno-occlusive disease (PVOD): If signs of pulmonary edema signs or symptoms of liver dysfunction or injury or increases in bilirubin occur, consider the diagnosis of associated PVOD and consider discontinuing ≥2 x ULN (2.4, 5.1). TRACLEER (5.5). Based on animal data, TRACLEER is likely to cause major birth defects if used • Decreased sperm counts (5.6). during pregnancy (4.1, 5.2, 8.1). • Decreases in hemoglobin and hematocrit: Monitor hemoglobin levels after • Must exclude pregnancy before and during treatment (2.1, 4.1, 8.1). -

2016 Annual Report
ANNUAL REPORT 2016 MARCH 2017 TO OUR SHAREHOLDERS ALEX GORSKY Chairman and Chief Executive Officer I’ve worked in the health care industry for Rather, true innovations are the result of WE ARE UNITED nearly 30 years. It’s been both an honor and collaboration. And that collaboration is AND INSPIRED a privilege to work for Johnson & Johnson, driven by a diversity of ideas, individuals BY OUR CREDO, a company that touches the lives of over and disciplines – working together toward WHICH RINGS a billion people every day, around the a common goal. AS TRUE TODAY world. As I look at today’s health care AS IT DID WHEN landscape, it’s incredibly clear that the Today, more than ever, the world needs IT WAS WRITTEN pace of change has never been greater, leaders who are committed to working MORE THAN 70 or frankly, more exciting. together to help bring improved health YEARS AGO. and wellness to every person in every Today’s rapid change brings both corner of the globe. As the world’s largest opportunities and risks for any company and most broadly based health care in health care, and we are prepared company, we are uniquely positioned to help to address both. There are significant transform global health care; to shine a light challenges to overcome, but the tools, the on the most important issues we are facing; insights, the technologies, the innovations to collaborate across boundaries and – both evolutions and revolutions – all borders; to uncover scientific insights and combine to make today one of the most ideas; and to dedicate resources towards promising times for human health and for creating tomorrow’s breakthroughs.