Proposal to Permit the Field Release of Genetically Engineered Diamondback Moth in New York
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ARTHROPOD COMMUNITIES and PASSERINE DIET: EFFECTS of SHRUB EXPANSION in WESTERN ALASKA by Molly Tankersley Mcdermott, B.A./B.S
Arthropod communities and passerine diet: effects of shrub expansion in Western Alaska Item Type Thesis Authors McDermott, Molly Tankersley Download date 26/09/2021 06:13:39 Link to Item http://hdl.handle.net/11122/7893 ARTHROPOD COMMUNITIES AND PASSERINE DIET: EFFECTS OF SHRUB EXPANSION IN WESTERN ALASKA By Molly Tankersley McDermott, B.A./B.S. A Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science in Biological Sciences University of Alaska Fairbanks August 2017 APPROVED: Pat Doak, Committee Chair Greg Breed, Committee Member Colleen Handel, Committee Member Christa Mulder, Committee Member Kris Hundertmark, Chair Department o f Biology and Wildlife Paul Layer, Dean College o f Natural Science and Mathematics Michael Castellini, Dean of the Graduate School ABSTRACT Across the Arctic, taller woody shrubs, particularly willow (Salix spp.), birch (Betula spp.), and alder (Alnus spp.), have been expanding rapidly onto tundra. Changes in vegetation structure can alter the physical habitat structure, thermal environment, and food available to arthropods, which play an important role in the structure and functioning of Arctic ecosystems. Not only do they provide key ecosystem services such as pollination and nutrient cycling, they are an essential food source for migratory birds. In this study I examined the relationships between the abundance, diversity, and community composition of arthropods and the height and cover of several shrub species across a tundra-shrub gradient in northwestern Alaska. To characterize nestling diet of common passerines that occupy this gradient, I used next-generation sequencing of fecal matter. Willow cover was strongly and consistently associated with abundance and biomass of arthropods and significant shifts in arthropod community composition and diversity. -

Untangling Taxonomy: a DNA Barcode Reference Library for Canadian Spiders
Molecular Ecology Resources (2016) 16, 325–341 doi: 10.1111/1755-0998.12444 Untangling taxonomy: a DNA barcode reference library for Canadian spiders GERGIN A. BLAGOEV, JEREMY R. DEWAARD, SUJEEVAN RATNASINGHAM, STEPHANIE L. DEWAARD, LIUQIONG LU, JAMES ROBERTSON, ANGELA C. TELFER and PAUL D. N. HEBERT Biodiversity Institute of Ontario, University of Guelph, Guelph, ON, Canada Abstract Approximately 1460 species of spiders have been reported from Canada, 3% of the global fauna. This study provides a DNA barcode reference library for 1018 of these species based upon the analysis of more than 30 000 specimens. The sequence results show a clear barcode gap in most cases with a mean intraspecific divergence of 0.78% vs. a min- imum nearest-neighbour (NN) distance averaging 7.85%. The sequences were assigned to 1359 Barcode index num- bers (BINs) with 1344 of these BINs composed of specimens belonging to a single currently recognized species. There was a perfect correspondence between BIN membership and a known species in 795 cases, while another 197 species were assigned to two or more BINs (556 in total). A few other species (26) were involved in BIN merges or in a combination of merges and splits. There was only a weak relationship between the number of specimens analysed for a species and its BIN count. However, three species were clear outliers with their specimens being placed in 11– 22 BINs. Although all BIN splits need further study to clarify the taxonomic status of the entities involved, DNA bar- codes discriminated 98% of the 1018 species. The present survey conservatively revealed 16 species new to science, 52 species new to Canada and major range extensions for 426 species. -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

Arachnids (Excluding Acarina and Pseudoscorpionida) of the Wichita Mountains Wildlife Refuge, Oklahoma
OCCASIONAL PAPERS THE MUSEUM TEXAS TECH UNIVERSITY NUMBER 67 5 SEPTEMBER 1980 ARACHNIDS (EXCLUDING ACARINA AND PSEUDOSCORPIONIDA) OF THE WICHITA MOUNTAINS WILDLIFE REFUGE, OKLAHOMA JAMES C. COKENDOLPHER AND FRANK D. BRYCE The Wichita Mountains are located in eastern Greer, southern Kiowa, and northwestern Comanche counties in Oklahoma. Since their formation more than 300 million years ago, these rugged mountains have been fragmented and weathered, until today the highest peak (Mount Pinchot) stands only 756 meters above sea level (Tyler, 1977). The mountains are composed predominantly of granite and gabbro. Forests of oak, elm, and walnut border most waterways, while at elevations from 153 to 427 meters prair ies are the predominant vegetation type. A more detailed sum mary of the climatic and biotic features of the Wichitas has been presented by Blair and Hubbell (1938). A large tract of land in the eastern range of the Wichita Moun tains (now northeastern Comanche County) was set aside as the Wichita National Forest by President McKinley during 1901. In 1905, President Theodore Roosevelt created a game preserve on those lands managed by the Forest Service. Since 1935, this pre serve has been known as the Wichita Mountains Wildlife Refuge. Numerous papers on Oklahoma spiders have been published (Bailey and Chada, 1968; Bailey et al., 1968; Banks et al, 1932; Branson, 1958, 1959, 1966, 1968; Branson and Drew, 1972; Gro- thaus, 1968; Harrel, 1962, 1965; Horner, 1975; Rogers and Horner, 1977), but only a single, comprehensive work (Banks et al., 1932) exists covering all arachnid orders in the state. Further additions and annotations to the arachnid fauna of Oklahoma can be found 2 OCCASIONAL PAPERS MUSEUM TEXAS TECH UNIVERSITY in recent revisionary studies. -

A Phylogenetic Study of the Mediterranean Genus Hormathophylla (Cruciferae: Alysseae) Based on Nuclear and Plastid Sequences
RESEARCH ARTICLE Plant evolution in alkaline magnesium-rich soils: A phylogenetic study of the Mediterranean genus Hormathophylla (Cruciferae: Alysseae) based on nuclear and plastid sequences Esteban Salmero n-SaÂnchez 1,2*, Javier Fuertes-Aguilar3, Stanislav SÏ paniel4,5, Francisco a1111111111 ID Javier PeÂrez-GarcõÂa1, Encarna Merlo1, Juan Antonio Garrido-Becerra1, Juan Mota1 a1111111111 a1111111111 1 Departamento de BiologõÂa y GeologõÂa, CEI.MAR and CECOUAL, Universidad de AlmerõÂa, AlmerõÂa, Spain, a1111111111 2 Departamento de BotaÂnica, Unidad de ConservacioÂn Vegetal, Universidad de Granada, Granada, Spain, a1111111111 3 Real JardõÂn BotaÂnico, CSIC, Madrid, Spain, 4 Institute of Botany, Plant Science and Biodiversity Centre, Slovak Academy of Sciences, Bratislava, Slovak Republic, 5 Department of Botany, Faculty of Science, Charles University in Prague, Prague, Czech Republic * [email protected] OPEN ACCESS Citation: SalmeroÂn-SaÂnchez E, Fuertes-Aguilar J, Abstract SÏpaniel S, PeÂrez-GarcõÂa FJ, Merlo E, Garrido- Becerra JA, et al. (2018) Plant evolution in alkaline Habitats with alkaline edaphic substrates are often associated with plant speciation and magnesium-rich soils: A phylogenetic study of the diversification. The tribe Alysseae, in the family Brassicaceae, epitomizes this evolutionary Mediterranean genus Hormathophylla (Cruciferae: Alysseae) based on nuclear and plastid sequences. trend. In this lineage, some genera, like Hormathophylla, can serve as a good case for test- PLoS ONE 13(12): e0208307. https://doi.org/ ing the evolutionary framework. This genus is centered in the western Mediterranean. It 10.1371/journal.pone.0208307 grows on different substrates, but mostly on alkaline soils. It has been suggested that diver- Editor: Dong Hoon Shin, Seoul National University sification in many lineages of the tribe Alysseae and in the genus Hormathophylla is linked College of Medicine, REPUBLIC OF KOREA to a tolerance for high levels of Mg+2 in xeric environments. -

Mesostigmata No
16 (1) · 2016 Christian, A. & K. Franke Mesostigmata No. 27 ............................................................................................................................................................................. 1 – 41 Acarological literature .................................................................................................................................................... 1 Publications 2016 ........................................................................................................................................................................................... 1 Publications 2015 ........................................................................................................................................................................................... 9 Publications, additions 2014 ....................................................................................................................................................................... 17 Publications, additions 2013 ....................................................................................................................................................................... 18 Publications, additions 2012 ....................................................................................................................................................................... 20 Publications, additions 2011 ...................................................................................................................................................................... -

Coleoptera: Coccinellidae) in the Palearctic Region
Oriental Insects ISSN: (Print) (Online) Journal homepage: https://www.tandfonline.com/loi/toin20 Review of the genus Hippodamia (Coleoptera: Coccinellidae) in the Palearctic region Amir Biranvand, Oldřich Nedvěd, Romain Nattier, Elizaveta Nepaeva & Danny Haelewaters To cite this article: Amir Biranvand, Oldřich Nedvěd, Romain Nattier, Elizaveta Nepaeva & Danny Haelewaters (2021) Review of the genus Hippodamia (Coleoptera: Coccinellidae) in the Palearctic region, Oriental Insects, 55:2, 293-304, DOI: 10.1080/00305316.2020.1763871 To link to this article: https://doi.org/10.1080/00305316.2020.1763871 Published online: 15 May 2020. Submit your article to this journal Article views: 87 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=toin20 ORIENTAL INSECTS 2021, VOL. 55, NO. 2, 293–304 https://doi.org/10.1080/00305316.2020.1763871 Review of the genus Hippodamia (Coleoptera: Coccinellidae) in the Palearctic region Amir Biranvanda, Oldřich Nedvěd b,c, Romain Nattierd, Elizaveta Nepaevae and Danny Haelewaters b aYoung Researchers and Elite Club, Khorramabad Branch, Islamic Azad University, Khorramabad, Iran; bFaculty of Science, University of South Bohemia, České Budějovice, Czech Republic; cBiology Centre, Czech Academy of Sciences, Institute of Entomology, České Budějovice, Czech Republic; dInstitut de Systématique, Evolution, Biodiversité, ISYEB, Muséum National d’Histoire Naturelle (MNHN), CNRS, Sorbonne Université, EPHE, Université des Antilles, Paris, France; eAltai State University, Barnaul, Russian Federation ABSTRACT ARTICLE HISTORY Hippodamia Chevrolat, 1836 currently comprises 19 species. Received 9 December 2019 Four species of Hippodamia are native to the Palearctic region: Accepted 29 April 2020 Hippodamia arctica (Schneider, 1792), H. -

Small White Brassicas #Wildflowerhour #Dinkymoira
Small White Brassicas #wildflowerhour #dinkymoira Plants with small, white, four-petalled flowers are popping up all over the place in pavement cracks and walls, on road verges and in flower beds …… What are they all and how can you tell them apart? These plants are members of the Brassicaceae, the Cabbage family. You may also hear them being referred to as crucifers (from the Latin for Danish Scurvygrass cross) which describes the cross-wise arrangement of the four Cochlearia danica petals. For this family one of the key identification features is their This plant has a most unhelpful common name, as it is not seed pods, which come in many different shapes and sizes. Here are Thale Cress a grass and has no particular association with Denmark! some of our early flowering, small, white-flowered brassicas. Arabidopsis thaliana It starts flowering around March and is a low-growing, mat Hairy Bittercress Wavy Bittercress Thale Cress can look a bit superficially similar to Shepherd’s forming plant with lilac-white flowers. The leaves are dark Cardamine hirsuta Cardamine flexuosa Purse (see below) but once the slender, cylindrical seed green - the basal ones are long-stalked, shallowly lobed Seed pods held pods appear the two are easily distinguished. Flowering and have a heart shaped base. The stem leaves are ivy- straight up from around March, it grows on bare and disturbed ground, shaped, and the upper ones are unstalked. The seed pods and also walls and pavement cracks. The leaves of the (not pictured) are egg-shaped. Traditionally a coastal plant, basal rosette have some forked or 3-rayed stellate hairs. -

Evolution and Diversification of the Plant Gibberellin Receptor GID1
Evolution and diversification of the plant gibberellin receptor GID1 Hideki Yoshidaa,b, Eiichi Tanimotoc, Takaaki Hiraia, Yohei Miyanoirid,e, Rie Mitania, Mayuko Kawamuraa, Mitsuhiro Takedad,f, Sayaka Takeharaa, Ko Hiranoa, Masatsune Kainoshod,g, Takashi Akagih, Makoto Matsuokaa,1, and Miyako Ueguchi-Tanakaa,1 aBioscience and Biotechnology Center, Nagoya University, Nagoya, 464-8601 Aichi, Japan; bKihara Institute for Biological Research, Yokohama City University, Yokohama, 244-0813 Kanagawa, Japan; cGraduate School of Natural Sciences, Nagoya City University, Nagoya, 467-8501 Aichi, Japan; dStructural Biology Research Center, Graduate School of Science, Nagoya University, Nagoya, 464-8601 Aichi, Japan; eResearch Center for State-of-the-Art Functional Protein Analysis, Institute for Protein Research, Osaka University, Suita, 565-0871 Osaka, Japan; fDepartment of Structural BioImaging, Faculty of Life Sciences, Kumamoto University, 862-0973 Kumamoto, Japan; gGraduate School of Science and Engineering, Tokyo Metropolitan University, Hachioji, 192-0397 Tokyo, Japan; and hGraduate School of Agriculture, Kyoto University, 606-8502 Kyoto, Japan Edited by Mark Estelle, University of California, San Diego, La Jolla, CA, and approved July 10, 2018 (received for review April 9, 2018) The plant gibberellin (GA) receptor GID1 shows sequence similarity erwort Marchantia polymorpha (5–7). Furthermore, Hirano et al. to carboxylesterase (CXE). Here, we report the molecular evolution (5) reported that GID1s in the lycophyte Selaginella moellen- of GID1 from establishment to functionally diverse forms in dorffii (SmGID1s) have unique properties in comparison with eudicots. By introducing 18 mutagenized rice GID1s into a rice angiosperm GID1s: namely, lower affinity to bioactive GAs and gid1 null mutant, we identified the amino acids crucial for higher affinity to inactive GAs (lower specificity). -

Toxicity, Sublethal and Low Dose Effects of Imidacloprid and Deltamethrin on the Aphidophagous Predator Ceratomegilla Undecimnotata (Coleoptera: Coccinellidae)
insects Article Toxicity, Sublethal and Low Dose Effects of Imidacloprid and Deltamethrin on the Aphidophagous Predator Ceratomegilla undecimnotata (Coleoptera: Coccinellidae) Panagiotis J. Skouras 1,* , Anastasios I. Darras 2 , Marina Mprokaki 1, Vasilios Demopoulos 3, John T. Margaritopoulos 4 , Costas Delis 2 and George J. Stathas 1 1 Laboratory of Agricultural Entomology and Zoology, Department of Agriculture, Kalamata Campus, University of the Peloponnese, 24100 Antikalamos, Greece; [email protected] (M.M.); [email protected] (G.J.S.) 2 Department of Agriculture, Kalamata Campus, University of the Peloponnese, 24100 Antikalamos, Greece; [email protected] (A.I.D.); [email protected] (C.D.) 3 Laboratory of Plant Protection, Department of Agriculture, Kalamata Campus, University of the Peloponnese, 24100 Antikalamos, Greece; [email protected] 4 Department of Plant Protection, Institute of Industrial and Fodder Crops, Hellenic Agricultural Organization “DEMETER”—NAGREF, 38446 Volos, Greece; [email protected] * Correspondence: [email protected]; Tel.: +30-27210-45277 Simple Summary: Chemical insecticides are used to control agricultural pests all over the world. However, extensive use of chemical insecticides can be harmful to human health and negatively Citation: Skouras, P.J.; Darras, A.I.; impact the environment and biological control agents. We studied the toxicity and sublethal effects Mprokaki, M.; Demopoulos, V.; of imidacloprid and deltamethrin on the aphidophagous coccinellid predator Ceratomegilla -

Species List For: Engelmann Woods NA 174 Species
Species List for: Engelmann Woods NA 174 Species Franklin County Date Participants Location NA List NA Nomination List List made by Maupin and Kurz, 9/9/80, and 4/21/93 WGNSS Lists Webster Groves Nature Study Society Fieldtrip Participants WGNSS Vascular Plant List maintained by Steve Turner Species Name (Synonym) Common Name Family COFC COFW Acalypha virginica Virginia copperleaf Euphorbiaceae 2 3 Acer negundo var. undetermined box elder Sapindaceae 1 0 Acer saccharum var. undetermined sugar maple Sapindaceae 5 3 Achillea millefolium yarrow Asteraceae/Anthemideae 1 3 Actaea pachypoda white baneberry Ranunculaceae 8 5 Adiantum pedatum var. pedatum northern maidenhair fern Pteridaceae Fern/Ally 6 1 Agastache nepetoides yellow giant hyssop Lamiaceae 4 3 Ageratina altissima var. altissima (Eupatorium rugosum) white snakeroot Asteraceae/Eupatorieae 2 3 Agrimonia rostellata woodland agrimony Rosaceae 4 3 Ambrosia artemisiifolia common ragweed Asteraceae/Heliantheae 0 3 Ambrosia trifida giant ragweed Asteraceae/Heliantheae 0 -1 Amelanchier arborea var. arborea downy serviceberry Rosaceae 6 3 Antennaria parlinii var. undetermined (A. plantaginifolia) plainleaf pussytoes Asteraceae/Gnaphalieae 5 5 Aplectrum hyemale putty root Orchidaceae 8 1 Aquilegia canadensis columbine Ranunculaceae 6 1 Arisaema triphyllum ssp. triphyllum (A. atrorubens) Jack-in-the-pulpit Araceae 6 -2 Aristolochia serpentaria Virginia snakeroot Aristolochiaceae 6 5 Arnoglossum atriplicifolium (Cacalia atriplicifolia) pale Indian plantain Asteraceae/Senecioneae 4 5 Arnoglossum reniforme (Cacalia muhlenbergii) great Indian plantain Asteraceae/Senecioneae 8 5 Asarum canadense wild ginger Aristolochiaceae 6 5 Asclepias quadrifolia whorled milkweed Asclepiadaceae 6 5 Asimina triloba pawpaw Annonaceae 5 0 Asplenium rhizophyllum (Camptosorus) walking fern Aspleniaceae Fern/Ally 7 5 Asplenium trichomanes ssp. trichomanes maidenhair spleenwort Aspleniaceae Fern/Ally 9 5 Srank: SU Grank: G? * Barbarea vulgaris yellow rocket Brassicaceae 0 0 Blephilia hirsuta var. -
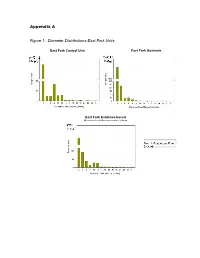
Appendix a Figure 1: Diameter Distributions-East Fork Units
Appendix A Figure 1: Diameter Distributions-East Fork Units East Fork Control Unit East Fork Goshawk Guidelines Unit East Fork Evidence-based Ecological Restoration Unit Figure 2: Diameter Distributions-Redondo Units Redondo Control Unit Redondo Goshawk Guidelines Unit Redondo Evidence-based Ecological Restoration Unit Figure 3: FlamMap Simulated Crown Fire Behavior: East Fork Project Area 20 mph Southwest Wind 25 mph 30 mph 35 mph 40 mph Map Legend Crown Fire Activity N No crown fire Passive crown activity fire (torching) Active crown fire (spreading) Important Note: Expected Crown Fire Behavior by Percent The 97th percentile of low fuel of Project Area moistures for June from 34 No Crown Passive years of data from the Kaibab Fire Crown Fire Active National Forest, Arizona were used in all simulations. This Windspeed Activity (torching) Crown Fire 20 79.9 0.9 19.2 input represents potential 25 71.2 1.9 26.9 extremes of fire weather during the peak of the fire 30 64.9 2.9 32.3 season, not average fire 35 57.3 4.7 38.0 season conditions. 40 48.7 9.4 42.0 Figure 4: FlamMap Simulated Crown Fire Behavior: Redondo Project Area 20 mph Southwest Wind 25 mph 30 mph 35 mph 40 mph Map Legend Crown Fire Activity N Highway 4 Valles Caldera No crown fire Passive crown activity entrance road fire (torching) Active crown fire (spreading) Important Note: Expected Crown Fire Behavior by Percent The 97th percentile of low fuel of Project Area moistures for June from 34 Passive years of data from the Kaibab No Crown Crown Fire Active Crown National Forest, Arizona were Windspeed Fire Activity (torching) Fire used in all simulations.