Increasing Signal Amplitude in Electrical Impedance Tomography of Neural Activity Using a Parallel Resistor Inductor Capacitor (RLC) Circuit
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary Physics (I-Introduction)
1 Glossary Physics (I-introduction) - Efficiency: The percent of the work put into a machine that is converted into useful work output; = work done / energy used [-]. = eta In machines: The work output of any machine cannot exceed the work input (<=100%); in an ideal machine, where no energy is transformed into heat: work(input) = work(output), =100%. Energy: The property of a system that enables it to do work. Conservation o. E.: Energy cannot be created or destroyed; it may be transformed from one form into another, but the total amount of energy never changes. Equilibrium: The state of an object when not acted upon by a net force or net torque; an object in equilibrium may be at rest or moving at uniform velocity - not accelerating. Mechanical E.: The state of an object or system of objects for which any impressed forces cancels to zero and no acceleration occurs. Dynamic E.: Object is moving without experiencing acceleration. Static E.: Object is at rest.F Force: The influence that can cause an object to be accelerated or retarded; is always in the direction of the net force, hence a vector quantity; the four elementary forces are: Electromagnetic F.: Is an attraction or repulsion G, gravit. const.6.672E-11[Nm2/kg2] between electric charges: d, distance [m] 2 2 2 2 F = 1/(40) (q1q2/d ) [(CC/m )(Nm /C )] = [N] m,M, mass [kg] Gravitational F.: Is a mutual attraction between all masses: q, charge [As] [C] 2 2 2 2 F = GmM/d [Nm /kg kg 1/m ] = [N] 0, dielectric constant Strong F.: (nuclear force) Acts within the nuclei of atoms: 8.854E-12 [C2/Nm2] [F/m] 2 2 2 2 2 F = 1/(40) (e /d ) [(CC/m )(Nm /C )] = [N] , 3.14 [-] Weak F.: Manifests itself in special reactions among elementary e, 1.60210 E-19 [As] [C] particles, such as the reaction that occur in radioactive decay. -

Oscillating Currents
Oscillating Currents • Ch.30: Induced E Fields: Faraday’s Law • Ch.30: RL Circuits • Ch.31: Oscillations and AC Circuits Review: Inductance • If the current through a coil of wire changes, there is an induced emf proportional to the rate of change of the current. •Define the proportionality constant to be the inductance L : di εεε === −−−L dt • SI unit of inductance is the henry (H). LC Circuit Oscillations Suppose we try to discharge a capacitor, using an inductor instead of a resistor: At time t=0 the capacitor has maximum charge and the current is zero. Later, current is increasing and capacitor’s charge is decreasing Oscillations (cont’d) What happens when q=0? Does I=0 also? No, because inductor does not allow sudden changes. In fact, q = 0 means i = maximum! So now, charge starts to build up on C again, but in the opposite direction! Textbook Figure 31-1 Energy is moving back and forth between C,L 1 2 1 2 UL === UB === 2 Li UC === UE === 2 q / C Textbook Figure 31-1 Mechanical Analogy • Looks like SHM (Ch. 15) Mass on spring. • Variable q is like x, distortion of spring. • Then i=dq/dt , like v=dx/dt , velocity of mass. By analogy with SHM, we can guess that q === Q cos(ωωω t) dq i === === −−−ωωωQ sin(ωωω t) dt Look at Guessed Solution dq q === Q cos(ωωω t) i === === −−−ωωωQ sin(ωωω t) dt q i Mathematical description of oscillations Note essential terminology: amplitude, phase, frequency, period, angular frequency. You MUST know what these words mean! If necessary review Chapters 10, 15. -
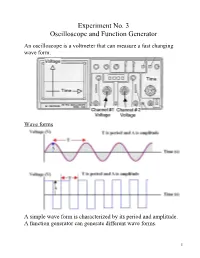
Experiment No. 3 Oscilloscope and Function Generator
Experiment No. 3 Oscilloscope and Function Generator An oscilloscope is a voltmeter that can measure a fast changing wave form. Wave forms A simple wave form is characterized by its period and amplitude. A function generator can generate different wave forms. 1 Part 1 of the experiment (together) Generate a square wave of frequency of 1.8 kHz with the function generator. Measure the amplitude and frequency with the Fluke DMM. Measure the amplitude and frequency with the oscilloscope. 2 The voltage amplitude has 2.4 divisions. Each division means 5.00 V. The amplitude is (5)(2.4) = 12.0 V. The Fluke reading was 11.2 V. The period (T) of the wave has 2.3 divisions. Each division means 250 s. The period is (2.3)(250 s) = 575 s. 1 1 f 1739 Hz The frequency of the wave = T 575 10 6 The Fluke reading was 1783 Hz The error is at least 0.05 divisions. The accuracy can be improved by displaying the wave form bigger. 3 Now, we have two amplitudes = 4.4 divisions. One amplitude = 2.2 divisions. Each division = 5.00 V. One amplitude = 11.0 V Fluke reading was 11.2 V T = 5.7 divisions. Each division = 100 s. T = 570 s. Frequency = 1754 Hz Fluke reading was 1783 Hz. It was always better to get a bigger display. 4 Part 2 of the experiment -Measuring signals from the DVD player. Next turn on the DVD Player and turn on the accessory device that is attached to the top of the DVD player. -

Peak and Root-Mean-Square Accelerations Radiated from Circular Cracks and Stress-Drop Associated with Seismic High-Frequency Radiation
J. Phys. Earth, 31, 225-249, 1983 PEAK AND ROOT-MEAN-SQUARE ACCELERATIONS RADIATED FROM CIRCULAR CRACKS AND STRESS-DROP ASSOCIATED WITH SEISMIC HIGH-FREQUENCY RADIATION Teruo YAMASHITA Earthquake Research Institute, the University of Tokyo, Tokyo, Japan (Received July 25, 1983) We derive an approximate expression for far-field spectral amplitude of acceleration radiated by circular cracks. The crack tip velocity is assumed to make abrupt changes, which can be the sources of high-frequency radiation, during the propagation of crack tip. This crack model will be usable as a source model for the study of high-frequency radiation. The expression for the spectral amplitude of acceleration is obtained in the following way. In the high-frequency range its expression is derived, with the aid of geometrical theory of diffraction, by extending the two-dimensional results. In the low-frequency range it is derived on the assumption that the source can be regarded as a point. Some plausible assumptions are made for its behavior in the intermediate- frequency range. Theoretical expressions for the root-mean-square and peak accelerations are derived by use of the spectral amplitude of acceleration obtained in the above way. Theoretically calculated accelerations are compared with observed ones. The observations are shown to be well explained by our source model if suitable stress-drop and crack tip velocity are assumed. Using Brune's model as an earthquake source model, Hanks and McGuire showed that the seismic ac- celerations are well predicted by a stress-drop which is higher than the statically determined stress-drop. However, their conclusion seems less reliable since Brune's source model cannot be applied to the study of high-frequency radiation. -

Amplitude-Probability Distributions for Atmospheric Radio Noise
S 7. •? ^ I ft* 2. 3 NBS MONOGRAPH 23 Amplitude-Probability Distributions for Atmospheric Radio Noise N v " X \t£ , " o# . .. » U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scientific and technical problems; inven- tion and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, develop- ment, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's work take the form of either actual equipment and devices or pub- -

Amplitude Modulation of the AD9850 Direct Digital Synthesizer by Richard Cushing, Applications Engineer
AN-423 a APPLICATION NOTE ONE TECHNOLOGY WAY • P.O. BOX 9106 • NORWOOD, MASSACHUSETTS 02062-9106 • 617/329-4700 Amplitude Modulation of the AD9850 Direct Digital Synthesizer by Richard Cushing, Applications Engineer This application note will offer a method to voltage con- The voltage at the RSET pin is part of the feedback loop of trol or amplitude modulate the output current of the the (internal) control amplifier and must not be exter- AD9850 DDS using an enhancement mode MOSFET to nally altered. The RSET modification circuit, Figure 2, replace the fixed RSET resistor; and a broadband RF uses Q1 as a variable resistor and R2 as a fixed current transformer to combine the DDS DAC outputs to pro- limit resistor in case Q1 is allowed to turn on too much. duce a symmetrical AM modulation envelope. Modula- C1 inhibits noise when Q1 is operated near cutoff. R1 tion with reasonable linearity is possible at rates lowers the input impedance for additional noise preven- exceeding 50 kHz. The AD9850 DDS output current tion. The input voltage to Q1 required to fully modulate (20 mA maximum) is normally set with a fixed resistor the AD9850 output is approximately 1.5 volts p-p and from the RSET (Pin 12) input to ground. The DAC outputs is dc offset by approximately 2.3 volts, see Figure 4. are unipolar and complementary (180 degrees out of TO RSET DC OR AUDIO phase) of each other. D C1 PIN 12 INPUT Q1 510pF Use of an enhancement mode MOSFET is in keeping G 2N7000* with the single supply concept. -

Amplitude Modulation Transmitter Design
Amplitude LAB Modulation 5 Transmitter Design Introduction The motivation behind this project is to design, implement, and test an Amplitude Modulation (AM) Transmitter. The Transmitter consists of a Balanced Modulator circuit which takes an audio signal stream from a Walkman and mixes it with a sinusoidal signal from a 1MHz oscillator. The resulting output is amplified by an output stage before being transmitted through a wire-antenna. AM Transmitter Floorplan: Design Goals Given the limited timeframe, we are providing you with the individual circuits that you need to design and construct. Fig. 1 shows the floorplan for the AM Transmitter. It consists of a Balanced Modulator which multiplies an audio fre- quency (20Hz to ~15kHz) signal with a 1MHz carrier frequency sinusoidal signal. The Balanced Modulator’s output is amplified by an output stage which drives an antenna (in this experiment, the antenna is just a 3” copper wire). The Balanced Modulator (Fig. 2) is essentially an analog multiplier: its time domain output signal, Vout(t) is linearly related to the product of the time domain input signals V1(t) (called the modulation signal) and V2(t) (called the carrier sig- nal). Its transfer function has the form: V ()t = k ⋅⋅V ()t V ()t out 1 2 The Balanced Modulator uses the principle of the dependence of the BJT’s transconductance, gm, on the emitter current bias. In order to demonstrate the principle, consider the load currents IL1 and IL2. From your knowledge of differ- ential amplifier operation, IL1 V1 I – I = g ⋅ V ≈ -------- ⋅ ------ L1 L2 m in VT 2 Also, the bias current IB in the differential amplifiers can be expressed as: V – 0.7 ≈ 2 IB ------------------ RE 1 Lab : Amplitude Modulation Transmitter Design Antenna Balanced O/P stage Walkman Modulator Amplifier 1MHz Oscillator Figure 1 — Amplitude Modulation Transmitter floorplan. -

EE301 – INTRO to AC and SINUSOIDS Learning Objectives
EE301 – INTRO TO AC AND SINUSOIDS Learning Objectives a. Compare AC and DC voltage and current sources as defined by voltage polarity, current direction and magnitude over time b. Define the basic sinusoidal wave equations and waveforms, and determine amplitude, peak to peak values, phase, period, frequency, and angular velocity c. Determine the instantaneous value of a sinusoidal waveform d. Graph sinusoidal wave equations as a function of time and angular velocity using degrees and radians e. Define effective / root mean squared values f. Define phase shift and determine phase differences between same frequency waveforms Alternating Current (AC) With the exception of short-term capacitor and inductor transients, all voltages and currents we have seen up to this point have been “DC”—i.e., fixed in magnitude. Now we shift our focus to “AC” voltage and current sources. AC sources (usually represented by lowercase e(t) or i(t)) have a sinusoidal waveform. For an AC voltage, for example, the voltage polarity changes every cycle. On the other hand, for an AC current, the current changes direction each cycle with the source voltage. Voltage and Current Conventions When e has a positive value, its actual polarity is the same as the reference polarity. When i has a positive value, its actual direction is the same as the reference arrow. 1 9/14/2016 EE301 – INTRO TO AC AND SINUSOIDS Sinusoids Since our ac waveforms (voltages and currents) are sinusoidal, we need to have a ready familiarity with the equation for a sinusoid. The horizontal scale, referred to as the “time scale” can represent degrees or time. -

Oscilloscope Fundamentals 03W-8605-4 Edu.Qxd 3/31/09 1:55 PM Page 2
03W-8605-4_edu.qxd 3/31/09 1:55 PM Page 1 Oscilloscope Fundamentals 03W-8605-4_edu.qxd 3/31/09 1:55 PM Page 2 Oscilloscope Fundamentals Table of Contents The Systems and Controls of an Oscilloscope .18 - 31 Vertical System and Controls . 19 Introduction . 4 Position and Volts per Division . 19 Signal Integrity . 5 - 6 Input Coupling . 19 Bandwidth Limit . 19 The Significance of Signal Integrity . 5 Bandwidth Enhancement . 20 Why is Signal Integrity a Problem? . 5 Horizontal System and Controls . 20 Viewing the Analog Orgins of Digital Signals . 6 Acquisition Controls . 20 The Oscilloscope . 7 - 11 Acquisition Modes . 20 Types of Acquisition Modes . 21 Understanding Waveforms & Waveform Measurements . .7 Starting and Stopping the Acquisition System . 21 Types of Waves . 8 Sampling . 22 Sine Waves . 9 Sampling Controls . 22 Square and Rectangular Waves . 9 Sampling Methods . 22 Sawtooth and Triangle Waves . 9 Real-time Sampling . 22 Step and Pulse Shapes . 9 Equivalent-time Sampling . 24 Periodic and Non-periodic Signals . 10 Position and Seconds per Division . 26 Synchronous and Asynchronous Signals . 10 Time Base Selections . 26 Complex Waves . 10 Zoom . 26 Eye Patterns . 10 XY Mode . 26 Constellation Diagrams . 11 Z Axis . 26 Waveform Measurements . .11 XYZ Mode . 26 Frequency and Period . .11 Trigger System and Controls . 27 Voltage . 11 Trigger Position . 28 Amplitude . 12 Trigger Level and Slope . 28 Phase . 12 Trigger Sources . 28 Waveform Measurements with Digital Oscilloscopes 12 Trigger Modes . 29 Trigger Coupling . 30 Types of Oscilloscopes . .13 - 17 Digital Oscilloscopes . 13 Trigger Holdoff . 30 Digital Storage Oscilloscopes . 14 Display System and Controls . 30 Digital Phosphor Oscilloscopes . -

Wavelength and Amplitude
Wavelength and Amplitude Waves transmit energy and travel through materials as vibrations. All waves transmit energy, not matter. Nearly all waves travel through matter. Waves are created when a source (force) creates a vibration. Vibrations in materials set up wave-like disturbances that spread away from the source of the disturbance. This means, of course, that every wave starts somewhere. wavelength: the Where is the source of the wave below? Can you explain distance between why the rope is creating a wave form? the peaks of a wave Waves can be compared by the way they behave. Waves have a repeating pattern that gives them a shape and length. These characteristics allow us to describe wave behavior and, therefore, categorize waves with our descriptions. Waves change their behavior as they travel through different types of matter. To be able to use these wave properties, we must first understand how each wave is measured. Do you see any characteristics in the waving rope above that might help us describe a wave? Wave behavior can be measured by the distance between peaks (wavelength), the size of the peak (amplitude), or the speed of the peaks (frequency). Sound and earthquake waves are examples. These and other waves move at different speeds in different materials. frequency: the rate at which a vibration amplitude: the size of the occurs that constitutes a wave peak of a wave 1 Wavelength and Amplitude Amplitude: The height of a wave Wavelength: The distance between adjacent crests Label each letter Trough: The lowest point of a wave above Crest: The highest point of a wave Waves are moving energy. -

Vibrational Amplitude Frequency Characteristics Analysis of a Controlled Nonlinear Meso-Scale Beam
actuators Article Vibrational Amplitude Frequency Characteristics Analysis of a Controlled Nonlinear Meso-Scale Beam Zu-Guang Ying 1,* and Yi-Qing Ni 2 1 Department of Mechanics, School of Aeronautics and Astronautics, Zhejiang University, Hangzhou 310027, China 2 Hong Kong Branch of National Rail Transit Electrification and Automation Engineering Technology Research Center, Department of Civil and Environmental Engineering, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong; [email protected] * Correspondence: [email protected] Abstract: Vibration response and amplitude frequency characteristics of a controlled nonlinear meso- scale beam under periodic loading are studied. A method including a general analytical expression for harmonic balance solution to periodic vibration and an updated cycle iteration algorithm for amplitude frequency relation of periodic response is developed. A vibration equation with the general expression of nonlinear terms for periodic response is derived and a general analytical expression for harmonic balance solution is obtained. An updated cycle iteration procedure is proposed to obtain amplitude frequency relation. Periodic vibration response with various frequencies can be calculated uniformly using the method. The method can take into account the effect of higher harmonic components on vibration response, and it is applicable to various periodic vibration analyses including principal resonance, super-harmonic resonance, and multiple stationary responses. Numerical results demonstrate that the developed method has good convergence and accuracy. The Citation: Ying, Z.-G.; Ni, Y.-Q. response amplitude should be determined by the periodic solution with multiple harmonic terms Vibrational Amplitude Frequency Characteristics Analysis of a instead of only the first harmonic term. The damping effect on response illustrates that vibration Controlled Nonlinear Meso-Scale responses of the nonlinear meso beam can be reduced by feedback control with certain damping gain. -

AC, DC and Electrical Signals
From http://www.kpsec.freeuk.com/acdc.htm © John Hewes 2006, The Electronics Club, www.kpsec.freeuk.com AC, DC and Electrical Signals AC means Alternating Current and DC means Direct Current. AC and DC are also used when referring to voltages and electrical signals which are not currents! For example: a 12V AC power supply has an alternating voltage (which will make an alternating current flow). An electrical signal is a voltage or current which conveys information, usually it means a voltage. The term can be used for any voltage or current in a circuit. Alternating Current (AC) Alternating Current (AC) flows one way, then the other way, continually reversing direction. An AC voltage is continually changing between positive (+) and negative (-). AC from a power supply The rate of changing direction is called the frequency This shape is called a sine wave. of the AC and it is measured in hertz (Hz) which is the number of forwards-backwards cycles per second. Mains electricity in the UK has a frequency of 50Hz. See below for more details of signal properties. An AC supply is suitable for powering some devices such as lamps and heaters but almost all electronic This triangular signal is AC because it changes circuits require a steady DC supply (see below). between positive (+) and negative (-). Direct Current (DC) 1 Direct Current (DC) always flows in the same direction, but it may increase and decrease. A DC voltage is always positive (or always negative), but it may increase and decrease. Electronic circuits normally require a steady DC supply which is constant at one value or a smooth DC supply Steady DC which has a small variation called ripple.