Krešimir Krnjevic´
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historia Dela Arquitectura Contemporánea Es Pan
JUAN DANIEL FULLAONDO MARÍA TERESA MUÑOZ HISTORIA DELA ARQUITECTURA CONTEMPORÁNEA ,...,, ES PAN OLA TOMO 111 Y ORFEO DESCIENDE MOLLY EDITORIAL © Juan Daniel Fullaondo © María Teresa Muñoz Prohibida la reproducción total o parcial sin la autorización de los autores. Portada¡ Montaje sobre una fotografía de María Teresa Muñoz (1996). ' . MOLLY EDITORIAL María Teresa Muñoz. c/ Príncipe de Vergara, 117. 28002 Madrid. I.S.B.N.: 84-922708-0-2 Dep. Legal: M-15340-1997 Impreso en España Fotocomposición e impresión: Tecnovic Arte Gráfico, S.L. Antonio Pérez, 8. Tel. 562 56 43. 28002 Madrid a Carlos Flores, a Bruno Zevi, y a José María Sastres 6 ÍNDICE NOTA PREVIA ...................................................................................... 9 PRÓLOGO.............................................................................................. 11 PRIMERA PARTE Los primeros disidentes.................................................................. 19 Seguimos con los primeros disidentes .... .. .. .. .. .. .. .... .. .. .. .. .... .. .. .. .. .. 25 Otras observaciones .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. 31 Más notas sobre la primera generación ........................................ 35 José Antonio Coderch . .. .. .. .. .. ... .. .. .. 41 Más sobre Coderch . .. .. .. .. 65 Miguel Fisac . .. .. .. .. .. .. .. .. .. .. 73 Una cierta, apresurada, recapitulación .. .... .. .. .. .. .... .. .. .. .. .. .. .. .. .. .. .... 93 Últimos suspiros teóricos . 99 Algunos comentarios -
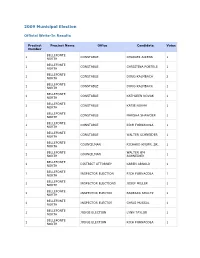
2009 Municipal Write-Ins
2009 Municipal Election Official Write-In Results Precinct Precinct Name Office Candidate Votes Number BELLEFONTE 1 CONSTABLE CHARLES AIKENS 1 NORTH BELLEFONTE 1 CONSTABLE CHRISTINA POBTELE 1 NORTH BELLEFONTE 1 CONSTABLE DOUG KALMBACH 2 NORTH BELLEFONTE 1 CONSTABLE DOUG KALMBACK 1 NORTH BELLEFONTE 1 CONSTABLE KATHLEEN NOVAK 1 NORTH BELLEFONTE 1 CONSTABLE KATIE NOVAK 1 NORTH BELLEFONTE 1 CONSTABLE MARSHA SHAWOER 1 NORTH BELLEFONTE 1 CONSTABLE RICH FORNACOLA 1 NORTH BELLEFONTE 1 CONSTABLE WALTER SCHNEIDER 1 NORTH BELLEFONTE 1 COUNCILMAN RICHARD KNUPP, SR. 1 NORTH BELLEFONTE WALTER GM 1 COUNCILMAN 1 NORTH SCHNEIDER BELLEFONTE 1 DISTRICT ATTORNEY KAREN ARNOLD 1 NORTH BELLEFONTE 1 INSPECTOR ELECTION RICH FORNACOLA 1 NORTH BELLEFONTE 1 INSPECTOR ELECTIONS JOSEF MILLER 1 NORTH BELLEFONTE 1 INSPECTOR ELECTOR BARBARA SHULTZ 1 NORTH BELLEFONTE 1 INSPECTOR ELECTOR CHRIS MUSSAL 1 NORTH BELLEFONTE 1 JUDGE ELECTION LYNN TAYLOR 1 NORTH BELLEFONTE 1 JUDGE ELECTION RICH FORNACOLA 1 NORTH BELLEFONTE 1 MAYOR BARRY SPICER, JR. 1 NORTH BELLEFONTE 1 TAX COLLECTOR BARRY SPICER, JR. 1 NORTH BELLEFONTE NORTH 2 BORO COUNCIL DANIAL REEDER 2 EAST BELLEFONTE NORTH 2 BORO COUNCILMAN BART SIMPSON 1 EAST BELLEFONTE NORTH 2 BORO COUNCILMAN GERALD REITZ 1 EAST BELLEFONTE NORTH 2 BORO COUNCILMAN KENT ADDIS 1 EAST BELLEFONTE NORTH 2 BORO COUNCILMAN MARTHA NASTASE 1 EAST BELLEFONTE NORTH 2 BORO COUNCILMAN RANDY BRACKBILL 1 EAST BELLEFONTE NORTH 2 CONSTABLE BENJAMIN COPOZI 1 EAST BELLEFONTE NORTH 2 CONSTABLE BRYAN SAMPSEL 1 EAST BELLEFONTE NORTH 2 CONSTABLE CASEY CRISSMAN-COX 1 EAST BELLEFONTE NORTH 2 CONSTABLE CRAIG MUNNELL 1 EAST BELLEFONTE NORTH 2 CONSTABLE DALE MOORE 1 EAST BELLEFONTE NORTH 2 CONSTABLE DANIAL REEDER 3 EAST BELLEFONTE NORTH 2 CONSTABLE DENIS O. -

Book of Abstracts: Studying Old Master Paintings
BOOK OF ABSTRACTS STUDYING OLD MASTER PAINTINGS TECHNOLOGY AND PRACTICE THE NATIONAL GALLERY TECHNICAL BULLETIN 30TH ANNIVERSARY CONFERENCE 1618 September 2009, Sainsbury Wing Theatre, National Gallery, London Supported by The Elizabeth Cayzer Charitable Trust STUDYING OLD MASTER PAINTINGS TECHNOLOGY AND PRACTICE THE NATIONAL GALLERY TECHNICAL BULLETIN 30TH ANNIVERSARY CONFERENCE BOOK OF ABSTRACTS 1618 September 2009 Sainsbury Wing Theatre, National Gallery, London The Proceedings of this Conference will be published by Archetype Publications, London in 2010 Contents Presentations Page Presentations (cont’d) Page The Paliotto by Guido da Siena from the Pinacoteca Nazionale of Siena 3 The rediscovery of sublimated arsenic sulphide pigments in painting 25 Marco Ciatti, Roberto Bellucci, Cecilia Frosinini, Linda Lucarelli, Luciano Sostegni, and polychromy: Applications of Raman microspectroscopy Camilla Fracassi, Carlo Lalli Günter Grundmann, Natalia Ivleva, Mark Richter, Heike Stege, Christoph Haisch Painting on parchment and panels: An exploration of Pacino di 5 The use of blue and green verditer in green colours in seventeenthcentury 27 Bonaguida’s technique Netherlandish painting practice Carole Namowicz, Catherine M. Schmidt, Christine Sciacca, Yvonne Szafran, Annelies van Loon, Lidwein Speleers Karen Trentelman, Nancy Turner Alterations in paintings: From noninvasive insitu assessment to 29 Technical similarities between mural painting and panel painting in 7 laboratory research the works of Giovanni da Milano: The Rinuccini -

Program of the 76Th Annual Meeting
PROGRAM OF THE 76 TH ANNUAL MEETING March 30−April 3, 2011 Sacramento, California THE ANNUAL MEETING of the Society for American Archaeology provides a forum for the dissemination of knowledge and discussion. The views expressed at the sessions are solely those of the speakers and the Society does not endorse, approve, or censor them. Descriptions of events and titles are those of the organizers, not the Society. Program of the 76th Annual Meeting Published by the Society for American Archaeology 900 Second Street NE, Suite 12 Washington DC 20002-3560 USA Tel: +1 202/789-8200 Fax: +1 202/789-0284 Email: [email protected] WWW: http://www.saa.org Copyright © 2011 Society for American Archaeology. All rights reserved. No part of this publication may be reprinted in any form or by any means without prior permission from the publisher. Program of the 76th Annual Meeting 3 Contents 4................ Awards Presentation & Annual Business Meeting Agenda 5………..….2011 Award Recipients 11.................Maps of the Hyatt Regency Sacramento, Sheraton Grand Sacramento, and the Sacramento Convention Center 17 ................Meeting Organizers, SAA Board of Directors, & SAA Staff 18 ............... General Information . 20. .............. Featured Sessions 22 ............... Summary Schedule 26 ............... A Word about the Sessions 28…………. Student Events 29………..…Sessions At A Glance (NEW!) 37................ Program 169................SAA Awards, Scholarships, & Fellowships 176................ Presidents of SAA . 176................ Annual Meeting Sites 178................ Exhibit Map 179................Exhibitor Directory 190................SAA Committees and Task Forces 194…….…….Index of Participants 4 Program of the 76th Annual Meeting Awards Presentation & Annual Business Meeting APRIL 1, 2011 5 PM Call to Order Call for Approval of Minutes of the 2010 Annual Business Meeting Remarks President Margaret W. -

Cumulated Bibliography of Biographies of Ocean Scientists Deborah Day, Scripps Institution of Oceanography Archives Revised December 3, 2001
Cumulated Bibliography of Biographies of Ocean Scientists Deborah Day, Scripps Institution of Oceanography Archives Revised December 3, 2001. Preface This bibliography attempts to list all substantial autobiographies, biographies, festschrifts and obituaries of prominent oceanographers, marine biologists, fisheries scientists, and other scientists who worked in the marine environment published in journals and books after 1922, the publication date of Herdman’s Founders of Oceanography. The bibliography does not include newspaper obituaries, government documents, or citations to brief entries in general biographical sources. Items are listed alphabetically by author, and then chronologically by date of publication under a legend that includes the full name of the individual, his/her date of birth in European style(day, month in roman numeral, year), followed by his/her place of birth, then his date of death and place of death. Entries are in author-editor style following the Chicago Manual of Style (Chicago and London: University of Chicago Press, 14th ed., 1993). Citations are annotated to list the language if it is not obvious from the text. Annotations will also indicate if the citation includes a list of the scientist’s papers, if there is a relationship between the author of the citation and the scientist, or if the citation is written for a particular audience. This bibliography of biographies of scientists of the sea is based on Jacqueline Carpine-Lancre’s bibliography of biographies first published annually beginning with issue 4 of the History of Oceanography Newsletter (September 1992). It was supplemented by a bibliography maintained by Eric L. Mills and citations in the biographical files of the Archives of the Scripps Institution of Oceanography, UCSD. -

Russian Museums Visit More Than 80 Million Visitors, 1/3 of Who Are Visitors Under 18
Moscow 4 There are more than 3000 museums (and about 72 000 museum workers) in Russian Moscow region 92 Federation, not including school and company museums. Every year Russian museums visit more than 80 million visitors, 1/3 of who are visitors under 18 There are about 650 individual and institutional members in ICOM Russia. During two last St. Petersburg 117 years ICOM Russia membership was rapidly increasing more than 20% (or about 100 new members) a year Northwestern region 160 You will find the information aboutICOM Russia members in this book. All members (individual and institutional) are divided in two big groups – Museums which are institutional members of ICOM or are represented by individual members and Organizations. All the museums in this book are distributed by regional principle. Organizations are structured in profile groups Central region 192 Volga river region 224 Many thanks to all the museums who offered their help and assistance in the making of this collection South of Russia 258 Special thanks to Urals 270 Museum creation and consulting Culture heritage security in Russia with 3M(tm)Novec(tm)1230 Siberia and Far East 284 © ICOM Russia, 2012 Organizations 322 © K. Novokhatko, A. Gnedovsky, N. Kazantseva, O. Guzewska – compiling, translation, editing, 2012 [email protected] www.icom.org.ru © Leo Tolstoy museum-estate “Yasnaya Polyana”, design, 2012 Moscow MOSCOW A. N. SCRiAbiN MEMORiAl Capital of Russia. Major political, economic, cultural, scientific, religious, financial, educational, and transportation center of Russia and the continent MUSEUM Highlights: First reference to Moscow dates from 1147 when Moscow was already a pretty big town. -

Sunnyvale Heritage Resources
CARIBBEAN DR 3RD AV G ST C ST BORDEAUX DR H ST 3RD AV Heritage Trees CARIBBEAN DR CASPIAN CT GENEVA DR ENTERPRISE WY 4TH AV Local Landmarks E ST CASPIAN DR BALTIC WY Heritage Resources 5TH AV JAVA DR 5TH AV MOFFETT PARK DR CROSSMAN AV 300-ft Buffer CHESAPEAKE TR GIBRALTAR CT GIBRALTAR DR ORLEANS DR MOFFETT PARK DR 7TH AV MACON RD ANVILWOOD City Boundary ENTERPRISEWY CT G ST C ST MOFFETT PARK CT 8TH AV HUMBOLDT CT PERSIAN DR FORGEWOODAV SR-237 ANVILWOODAV INNSBRUCK DR ELKO DR 9TH AV E ST FAIR OAKS WY BORREGAS AV D ST P O R P O I S ALDERWOODAV 11TH AV MOFFETT PARK DR E BA Y TR PARIA BIRCHWOODDR MATHILDA AV GLIESSEN JAEGALS RD GLIN SR-237 PLAZA DR PLENTYGLIN LA ROCHELLE TR TASMAN DR ENTERPRISE WY ENTERPRISE MONTEGO VIENNA DR KASSEL INNOVATION WAY BRADFORD DR MOLUCCA MONTEREY LEYTE MORSE AV KIHOLO LEMANS ROSS DR MUNICH LUND TASMAN CT KARLSTAD DR ESSEX AV COLTON AV FULTON AV DUNCAN AV HAMLIN CT SAGINAW FAIR OAKS AV TOYAMA DR SACO LAWRENCEEXPRESSWAY GARNER DR LYON US-101 SALERNO SAN JORGEKOSTANZ TIMOR KIEL CT SIRTE SOLOMON SUEZ LAKEBIRD DR CT DRIFTWOOD DRIFTWOOD CT CHARMWOOD CHARMWOOD CT SKYLAKE VALELAKE CT CT CLYDE AV BREEZEWOOD CT LAKECHIME DR JENNA PECOS WY AHWANEE AV LAKEDALE WY WEDDELL LOTUSLAKE CT GREENLAKE DR HIDDENLAKE DR WEDDELL DR MEADOWLAKE DR ALMANOR AV FAIRWOODAV STONYLAKE SR-237 LAKEFAIR DR CT CT LYRELAKE LYRELAKE HEM BLAZINGWOOD DR REDROCK CT LO CT CK ALTURAS AV SILVERLAKEDR AV CT CANDLEWOOD LAKEHAVEN DR BURNTWOOD CT C B LAKEHAVEN A TR U JADELAKE SAN ALESO AV R N MADRONE AV LAKEKNOLL DR N D T L PALOMAR AV SANTA CHRISTINA W CT -

Moonlite: a UK-Led Mission to the Moon Downloaded from by Guest on 24 September 2021
CRAWFORD, SMITH: MOONLITE MoonLITE: A UK-led mission to the Moon Downloaded from https://academic.oup.com/astrogeo/article/49/3/3.11/218588 by guest on 24 September 2021 Ian 1: Farside view of the Moon Crawford as seen by the and Alan Clementine spacecraft. Smith Penetrators discuss the launched by the MoonLITE orbiter scientific would allow surface objectives of investigations in areas not visited by Luna, the proposed Surveyor or Apollo missions. MoonLITE mission. (NASA/JPL/USGS) hile the surface missions to during the Apollo programme (see Wiec- the Moon of the 1960s and 1970s zorek et al. 2006, for a review). Moreover, the Wachieved a great deal, scientifically recent remote-sensing missions have themselves much was also left unresolved. The recent ABSTRACT raised questions that will require new surface plethora of lunar missions (flown or proposed) measurements for their resolution, of which reflects a resurgence in interest in the Moon, not MoonLITE is a proposal for a UK-led one of the most important is the circumstantial only in its own right, but also as a recorder of mission to the Moon that will place four evidence for water ice, and by implication other the early history of the Earth–Moon system and penetrators in the lunar surface in order volatiles, within permanently shaded craters at of the interplanetary environment 1 AU from the to make geochemical and geophysical the lunar poles (Feldman et al. 1998). Sun (e.g. Spudis 1996, Crawford 2004, Jolliff measurements that are impossible from In order to make significant further progress et al. -

At NALC's Doorstep
Volume 134/Number 2 February 2021 In this issue President’s Message 1 Branch Election Notices 81 Special issue LETTER CARRIER POLITICAL FUND The monthly journal of the NATIONAL ASSOCIATION OF LETTER CARRIERS ANARCHY at NALC’s doorstep— PAGE 1 { InstallInstall thethe freefree NALCNALC MemberMember AppApp forfor youryour iPhoneiPhone oror AndroidAndroid smartphonesmartphone As technology increases our ability to communicate, NALC must stay ahead of the curve. We’ve now taken the next step with the NALC Member App for iPhone and Android smartphones. The app was de- veloped with the needs of letter carriers in mind. The app’s features include: • Workplace resources, including the National • Instantaneous NALC news with Agreement, JCAM, MRS and CCA resources personalized push notifications • Interactive Non-Scheduled Days calendar and social media access • Legislative tools, including bill tracker, • Much more individualized congressional representatives and PAC information GoGo to to the the App App Store Store oror GoogleGoogle Play Play and and search search forfor “NALC “NALC Member Member App”App” toto install install for for free free President’s Message Anarchy on NALC’s doorstep have always taken great These developments have left our nation shaken. Our polit- pride in the NALC’s head- ical divisions are raw, and there now is great uncertainty about quarters, the Vincent R. the future. This will certainly complicate our efforts to advance Sombrotto Building. It sits our legislative agenda in the now-restored U.S. Capitol. But kitty-corner to the United there is reason for hope. IStates Capitol, a magnificent First, we should take solace in the fact that the attack on our and inspiring structure that has democracy utterly failed. -

Ertising in Science in School · Choose Between Advertising in the Quarterly Print Journal Or on Our Website
How many schools Spring 2011 Issue 18 and teachers do you reach – worldwide? In this issue: Biomimetics: clingy as an octopus or slick as a lotus leaf? Also: News from the EIROs: Mars, snakes, robots and DNA Advertising in Science in School · Choose between advertising in the quarterly print journal or on our website. · Website: reach over 30 000 science educators worldwide – every month. · In print: target up to 15 000 European science educators every quarter, including 3000 named subscribers. · Distribute your flyers, brochures, CD-ROMs or other materials either to 3000 named subscribers or to all recipients of the print copies. For more details, see www.scienceinschool.org/advertising Published by EIROforum: I S S N : 1 Initially supported by 8 1 Subscribe (free in Europe): www.scienceinschool.org 8 the European Union: - Highlighting the best in science teaching and research 0 3 5 3 sis_18_RZ_.qxq:Layout 1 15.03.2011 18:08 Uhr Seite B About Science in School Science in School promotes inspiring science teaching by encouraging communication between Editorial teachers, scientists and everyone else involved in European science education. The journal addresses science teaching both across Europe and across disciplines: highlighting the best in teaching and cutting-edge research. It covers not only biology, physics and chemistry, but also earth sciences, engineering and medicine, Happy birthday, focusing on interdisciplinary work. The contents include teaching materials; cutting-edge science; interviews with young scientists and inspiring Science in School! teachers; reviews of books and other resources; and European events for teachers and schools. Science in School is published quarterly, both online his issue of Science in School is rather special: it’s now and in print. -

She Smiles Sadly*.•
Number 5 Volume XXVIII. SEATTLE, WASHINGTON, FEBRUARY 4, 1933 SHE SMILES SADLY*.• • Kwan - Yin, Chinese Goddess of Mercy, some times called the Goddess of Peace, has reason these days for that sardonic expression, although the mocking smile is by no means a new one; she has worn it since the Wei Dynasty, Fifth Century A. D. The Goddess is the property of the Bos ton Museum of Art.— Courtesy The Art Digest. Featured This Week: Stuffed Zoos, by Dr. Herbert H. Gowen "Two Can Play"—, by Mack Mathews Editorials: (Up Hill and Down, Amateur Orchestra In Dissent, by George Pampel Starts, C's and R's, France Buys American) A Woman's Span (A Lyrical Sequence), by Helen Maring two THE TOWN CRIER FEBRUARY 4, 1933 By John Locke Worcester. Illus Stage trated with lantern slides. Puget "In Abraham's Bosom'' (Repertory Sound Academy of Science. Gug Playhouse)—Paul Green's Pulit AROUND THE TOWN genheim Hall. Wednesday, Febru zer prize drama produced by Rep ary 22, 8:15 p. m. ertory Company, with cast of Se attle negro actors. Direction Flor By MARGARET CALLAHAN Radio Highlights . , ence Bean James. A negro chorus sings spirituals. Wednesdays and Young People's Symphony Concert Fridays for limited run. 8:30 p.m. "Camille" (Repertory Playhouse) — Spanish ballroom, The Olympic. —New York Philharmonic, under direction of Bruno Walter. 8:30- "Funny Man" (Repertory Play All-University drama. February February 7, 8:30 p. m. 16 and 18, 8:30 p. m. Violin, piano trio—Jean Margaret 9:15 a. m. Saturday. KOL. house)—Comedy of old time Blue Danube—Viennese music un vaudeville life by Felix von Bres- Crow and Nora Crow Winkler, violinists, and Helen Louise Oles, der direction Dr. -

Fantasy & Science Fiction V030n04
THE MA GAZINE Of Fantasy and JACK VANCE Science Fiction ISAAC ASIMOV J.T. MCINTOS NOVELETS We Can Remember It For You Wholesale Philip k. dick 4 The Sorcerer Pharesm JACK VANCE 79 SHORT STORIES Appoggiatura A. M, MARPLE 25 But Soft, What Light . CAROL EMSHWILLER 41 The Sudden Silence J. T. MCINTOSH 45 The Face Is Familiar GILBERT THOMAS 64 The Space Twins JAMES PULLEY 75 Bordered In Black LARRY NIVEN 112 FEATURES Cartoon GAHAN WILSON 24 Books JUDITH MERRIL 31 Injected Memory THEODORE L. THOMAS 62 Verse: The Octopus DORIS PITKIN BUCK 63 Science: The Nobelmen of Science ISAAC ASIMOV 101 F&SF Marketplace 129 Cover by Jack Gaughan (illustrating "The Sorcerer Pharesm”) Joseph W. Ferman, publishek Edward L. Ferman, editor Ted White, assistant editor Isaac Asimov, science editor Judith Merril, book editor Robert P. Mills, consulting editor Dale Beardale, aRCULATiON manager The Magazine of Fantasy and Science Fiction, Volume 30, No. 4, Whole No. 179, Apr. 1966. Published monthly by Mercury Press, Inc., at 504 o copy. Annual subscription $5.00; $5.50 in Canada and the Pan American Union, $6.00 in all other countries. Publication office, 10 Ferry Street, Concord, N. H. 03302. Editorial and general mail should be sent to 347 East 53rd St., New York, N. Y. 10022. Second Class postage paid at Concord, N. H. Printed in U.S.A. © 1966 by Mercury Press, Inc. All rights including translations into other languages, reserved. Submissions must be accompanied by stamped, self-addressed envelopes: the Publisher assumes no responsibility for return of unsolicited manuscripts.