Koç, G.: First Molecular Characterization of the Partial Coat Protein and 3'Utr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding the Origin and Rapid Diversification of the Genus Anthurium Schott (Araceae), Integrating Molecular Phylogenetics, Morphology and Fossils
University of Missouri, St. Louis IRL @ UMSL Dissertations UMSL Graduate Works 8-3-2011 Understanding the origin and rapid diversification of the genus Anthurium Schott (Araceae), integrating molecular phylogenetics, morphology and fossils Monica Maria Carlsen University of Missouri-St. Louis, [email protected] Follow this and additional works at: https://irl.umsl.edu/dissertation Part of the Biology Commons Recommended Citation Carlsen, Monica Maria, "Understanding the origin and rapid diversification of the genus Anthurium Schott (Araceae), integrating molecular phylogenetics, morphology and fossils" (2011). Dissertations. 414. https://irl.umsl.edu/dissertation/414 This Dissertation is brought to you for free and open access by the UMSL Graduate Works at IRL @ UMSL. It has been accepted for inclusion in Dissertations by an authorized administrator of IRL @ UMSL. For more information, please contact [email protected]. Mónica M. Carlsen M.S., Biology, University of Missouri - St. Louis, 2003 B.S., Biology, Universidad Central de Venezuela – Caracas, 1998 A Thesis Submitted to The Graduate School at the University of Missouri – St. Louis in partial fulfillment of the requirements for the degree Doctor of Philosophy in Biology with emphasis in Ecology, Evolution and Systematics June 2011 Advisory Committee Peter Stevens, Ph.D. (Advisor) Thomas B. Croat, Ph.D. (Co-advisor) Elizabeth Kellogg, Ph.D. Peter M. Richardson, Ph.D. Simon J. Mayo, Ph.D Copyright, Mónica M. Carlsen, 2011 Understanding the origin and rapid diversification of the genus Anthurium Schott (Araceae), integrating molecular phylogenetics, morphology and fossils Mónica M. Carlsen M.S., Biology, University of Missouri - St. Louis, 2003 B.S., Biology, Universidad Central de Venezuela – Caracas, 1998 Advisory Committee Peter Stevens, Ph.D. -

Ornamental Garden Plants of the Guianas, Part 3
; Fig. 170. Solandra longiflora (Solanaceae). 7. Solanum Linnaeus Annual or perennial, armed or unarmed herbs, shrubs, vines or trees. Leaves alternate, simple or compound, sessile or petiolate. Inflorescence an axillary, extra-axillary or terminal raceme, cyme, corymb or panicle. Flowers regular, or sometimes irregular; calyx (4-) 5 (-10)- toothed; corolla rotate, 5 (-6)-lobed. Stamens 5, exserted; anthers united over the style, dehiscing by 2 apical pores. Fruit a 2-celled berry; seeds numerous, reniform. Key to Species 1. Trees or shrubs; stems armed with spines; leaves simple or lobed, not pinnately compound; inflorescence a raceme 1. S. macranthum 1. Vines; stems unarmed; leaves pinnately compound; inflorescence a panicle 2. S. seaforthianum 1. Solanum macranthum Dunal, Solanorum Generumque Affinium Synopsis 43 (1816). AARDAPPELBOOM (Surinam); POTATO TREE. Shrub or tree to 9 m; stems and leaves spiny, pubescent. Leaves simple, toothed or up to 10-lobed, to 40 cm. Inflorescence a 7- to 12-flowered raceme. Corolla 5- or 6-lobed, bluish-purple, to 6.3 cm wide. Range: Brazil. Grown as an ornamental in Surinam (Ostendorf, 1962). 2. Solanum seaforthianum Andrews, Botanists Repository 8(104): t.504 (1808). POTATO CREEPER. Vine to 6 m, with petiole-tendrils; stems and leaves unarmed, glabrous. Leaves pinnately compound with 3-9 leaflets, to 20 cm. Inflorescence a many- flowered panicle. Corolla 5-lobed, blue, purple or pinkish, to 5 cm wide. Range:South America. Grown as an ornamental in Surinam (Ostendorf, 1962). Sterculiaceae Monoecious, dioecious or polygamous trees and shrubs. Leaves alternate, simple to palmately compound, petiolate. Inflorescence an axillary panicle, raceme, cyme or thyrse. -

Plant Names Catalog 2013 1
Plant Names Catalog 2013 NAME COMMON NAME FAMILY PLOT Abildgaardia ovata flatspike sedge CYPERACEAE Plot 97b Acacia choriophylla cinnecord FABACEAE Plot 199:Plot 19b:Plot 50 Acacia cornigera bull-horn acacia FABACEAE Plot 50 Acacia farnesiana sweet acacia FABACEAE Plot 153a Acacia huarango FABACEAE Plot 153b Acacia macracantha steel acacia FABACEAE Plot 164 Plot 176a:Plot 176b:Plot 3a:Plot Acacia pinetorum pineland acacia FABACEAE 97b Acacia sp. FABACEAE Plot 57a Acacia tortuosa poponax FABACEAE Plot 3a Acalypha hispida chenille plant EUPHORBIACEAE Plot 4:Plot 41a Acalypha hispida 'Alba' white chenille plant EUPHORBIACEAE Plot 4 Acalypha 'Inferno' EUPHORBIACEAE Plot 41a Acalypha siamensis EUPHORBIACEAE Plot 50 'Firestorm' Acalypha siamensis EUPHORBIACEAE Plot 50 'Kilauea' Acalypha sp. EUPHORBIACEAE Plot 138b Acanthocereus sp. CACTACEAE Plot 138a:Plot 164 Acanthocereus barbed wire cereus CACTACEAE Plot 199 tetragonus Acanthophoenix rubra ARECACEAE Plot 149:Plot 71c Acanthus sp. ACANTHACEAE Plot 50 Acer rubrum red maple ACERACEAE Plot 64 Acnistus arborescens wild tree tobacco SOLANACEAE Plot 128a:Plot 143 1 Plant Names Catalog 2013 NAME COMMON NAME FAMILY PLOT Plot 121:Plot 161:Plot 204:Plot paurotis 61:Plot 62:Plot 67:Plot 69:Plot Acoelorrhaphe wrightii ARECACEAE palm:Everglades palm 71a:Plot 72:Plot 76:Plot 78:Plot 81 Acrocarpus fraxinifolius shingle tree:pink cedar FABACEAE Plot 131:Plot 133:Plot 152 Acrocomia aculeata gru-gru ARECACEAE Plot 102:Plot 169 Acrocomia crispa ARECACEAE Plot 101b:Plot 102 Acrostichum aureum golden leather fern ADIANTACEAE Plot 203 Acrostichum Plot 195:Plot 204:Plot 3b:Plot leather fern ADIANTACEAE danaeifolium 63:Plot 69 Actephila ovalis PHYLLANTHACEAE Plot 151 Actinorhytis calapparia calappa palm ARECACEAE Plot 132:Plot 71c Adansonia digitata baobab MALVACEAE Plot 112:Plot 153b:Plot 3b Adansonia fony var. -

Fairchild's Orchid Program
winter 2016 Fairchild’s Orchid Program: The synergy of science education, outreach and the beauty of the world’s most coveted plant published by fairchild tropical botanic garden The Shop AT FAIRCHILD Botanical Bird Glass Plate Regular price, $18.00 Member price, $16.20 GARDENING SUPPLIES | UNIQUE TROPICAL GIFTS | APPAREL ECO-FRIENDLY AND FAIR-TraDE PRODUCTS | ACCESSORIES | BOOKS TROPICAL GOURMET FOODS | HOME DÉCOR | ORCHIDS AND MUCH MORE Shop Hours: 7:30 a.m. - 5:30 p.m. Shop online at store.fairchildonline.com fairchild tropical botanic garden Photo by Rey Longchamp/FTBG contents FEATURES PARTNERS IN PLANT OAKES AMES: A shy man with a whip- CONSERVATION HALF 26 43 sharp sense of humor matched only by his A WORLD AWAY and wife Blanche’s passion for orchids DEPARTMENTS 4 FROM THE DIRECTOR 5 FROM THE CHIEF OPERATING OFFICER 7 SCHEDULE OF EVENTS 9 GET IN ON THE CONSERVATION 11 EXPLAINING 15 VIS-A-VIS VOLUNTEERS 16 WHAT’S BLOOMING 19 THE ART IN GARTEN 26 CONSERVING 31 BOOK REVIEW 39 WHAT’S IN STORE 41 PLANT SOCIETIES 50 WHAT’S IN A NAME 52 EDIBLE GARDENING 57 BUG BEAT 58 PLANT COLLECTIONS 62 FROM THE ARCHIVES 64 GARDEN VIEWS CREATING A GARDEN CITY IN SINGAPORE 32 from the director reycinetia cumingiana, a spectacular plant in our Tropical Plant Conservatory, comes from a mountaintop rainforest in the south of Luzon, Philippines. A relative of Fthe pandan, Freycinetia is a distant cousin of palms, grasses and bromeliads. In the Garden, F. cumingiana bursts into bloom in the shortest days of winter, just as it does in its native habitat. -

61 Floristic and Phytogeographic Aspects of Araceae in Cerro Pirre
FLORISTIC AND PHYTOGEOGRAPHIC ASPECTS OF ARACEAE IN CERRO PIRRE (DARIÉN, PANAMA) ORLANDO O. ORTIZ 1, MARÍA S. DE STAPF 1, 2, RICCARDO M. BALDINI 3 AND THOMAS B. CROAT 4 1Herbario PMA, Universidad de Panamá, Estafeta Universitaria, Ciudad de Panamá, Panamá. 2Departamento de Botánica, Universidad de Panamá, Estafeta Universitaria, Ciudad de Panamá, Panamá. 3Centro Studi Erbario Tropicale (FT herbarium) and Dipartimento di Biologia, Università di Firenze, Via La Pira 4, 50121, Firenze, Italy 4Missouri Botanical Garden, 4344 Shaw Blvd., St. Louis, MO 63110, USA. Correspondence author: Orlando O. Ortiz, [email protected] SUMMARY The aroid flora in Panama includes 436 described species in 26 genera, representing the richest country of Araceae in Central America. Much of the existing knowledge of the Panamanian aroids has been generated in the last 50 years, mainly due to extensive taxonomic studies and, to a lesser extent, by floristic studies. Floristic studies generated valuable information to better understand biodiversity, especially in the poorly- explored areas. For this reason, the main objective of this work is to study the floristic composition of the aroids of a botanically important region: Cerro Pirre (Darién Province). As a result, 430 specimens were Scientia, Vol. 28, N° 2 61 studied, comprising 94 species in 12 genera. The Aroid flora of Cerro Pirre is formed by species of wide geographic distribution (53%) and, to a lesser extent, endemic species (27%). Of the total species, approximately 43% are nomadic vines, 33% epiphytes, 23% terrestrial and a single species epilithic (1%). Ten new records for the flora of Cerro Pirre were recorded and one new record for Panama. -

Phantasea Tropical Botanical Garden
Phantasea Tropical Botanical Garden March/April 2015 Volumn 1, Issue 2 What’s Happening It’s springtime in Inside this issue: the garden! New interpretive signage is always being added and installed through- What’s Happening 1 The orchids are blooming now out the garden along with new indi- throughout the garden. Several of vidual plant name tags. the large lavender cattleyas are What’s New 1 Our website is now up and running showing off their beauty up in so check back often to: http:// trees. The phalaenopsis www.stthomasbotanicalgarden.com What’s Blooming 2 orchids are blooming throughout tasea_Tropical_Botanical_Garde for other news and happenings. the garden. More Blooming 2-3 You can also “Like Us” on facebook The bromeliads are starting to at: http://www.facebook.com/ Upcoming Events 3 bloom and show off all their stthomasbotanicalgarden and keep strange and unusual shapes and up with what’s in bloom on a con- color combinations. tinuing basis. Tropical Treats 3 The heliconias are starting to And we are now listed on TripAdvi- Featured Plant 4 peek out with their new blooms sor. If you have had a chance to in reds and yellows and the sugar visit us, a review is greatly appreci- birds and hummingbirds are ated. anxiously awaiting their opening http://www.tripadvisor.ca/ what is a botanica l since they love to drink the nectar Attraction_Review-g147404- garden ? from their flowers. d7701018-Reviews- Phan- A garden where a large variety of plants are culti- vated for exhibition and scientific purposes. Plants in a Botanical Gar- den are documented and What’s new: labeled. -

Inventarisasi Tumbuhan Angiospermae Di Lingkungan Man 5 Aceh Besar Sebagai Media Pembelajaran Pada Sub Pokok Bahasan Spermatophyta
INVENTARISASI TUMBUHAN ANGIOSPERMAE DI LINGKUNGAN MAN 5 ACEH BESAR SEBAGAI MEDIA PEMBELAJARAN PADA SUB POKOK BAHASAN SPERMATOPHYTA SKRIPSI Diajukan Oleh: WARDATI NIM. 150207028 Mahasiswa Fakultas Tarbiyah dan Keguruan Prodi Pendidikan Biologi FAKULTAS TARBIYAH DAN KEGURUAN UNIVERSITAS ISLAM NEGERI AR-RANIRY DARUSSALAM – BANDA ACEH 2020 ABSTRAK Proses pembelajaran biologi sub pokok bahasan spermatophyta di MAN 5 Aceh Besar selama ini siswa mendengar sambil mencatat penjelasan dari guru, siswa tidak pernah melihat langsung jenis tumbuhan yang terdapat di lingkungan, padahal jenis tumbuhan yang dijelaskan oleh guru sangat banyak. Hal ini menyebabkan siswa sering merasa bosan dan tidak tertarik belajar. Guru mata pelajaran biologi menyatakan bahwa tumbuhan yang terdapat di lingkungan tersebut tidak dimanfaatkan sebagai media pembelajaran dikarenakan tidak media pembelajaran yang membahas tentang tumbuhan yang ada lingkungan tersebut, sehingga tidak diketahui jenis-jenis tumbuhannya. Penelitian ini bertujuan untuk mengetahui jenis- jenis tumbuhan angiospermae apa saja yang terdapat di lingkungan di MAN 5 Aceh Besar, memanfaatkan hasil penelitian sebagai media pembelajaran sub pokok bahasan spermatophyta, mengetahui kelayakan output dari hasil penelitian dan untuk mengetahui respon siswa terhadap ouput dari penelitian. Rancangan penelitian yang digunakan deskriptif kuantitatif. Objek penelitian ini adalah semua tumbuhan angiospermae yang terdapat di lingkungan sekolah. Hasil penelitian menunjukkan bahwa terdapat 39 jenis dari 23 familia dengan total 241 individu. Output hasil penelitian berbentuk buku saku. Hasil uji kelayakan output penelitian diperoleh 84.69% dengan kategori sangat layak. Hasil respon siswa terhadap output penelitian diperoleh 83% termasuk dalam kategori positif. Kata Kunci: Inventarisasi, Angiospermae dan Buku Saku. v KATA PENGANTAR Puji syukur penulis panjatkan kehadirat Allah SWT atas segala rahmat, hidayah dan kemudahan yang selalu diberikan kepada hamba-Nya. -

History and Current Status of Systematic Research with Araceae Thomas B. Croat Missouri Botanical Garden P. O. Box 299 St. L
History and Current Status of Systematic Research with Araceae Thomas B. Croat Missouri Botanical Garden P. O. Box 299 St. Louis, MO 63166 U.S.A. Note: This paper, originally published in Aroideana Vol. 21, pp. 26-145 in 1998, is periodically updated on the IAS Web site with current additions. Any mistakes, proposed changes, or new publications that deal with the systematics of Araceae should be brought to my attention. Mail me at the address listed above, or E-mail me at [email protected]. Last revised: November 1, 2002. Copyright 2002 by Thomas B. Croat. Introduction The history of systematic work with Araceae has been previously covered by Nicolson (1987b), and was the subject of a chapter in the Genera of Araceae by Mayo, Bogner and Boyce (1997) and in Curtis's Botanical Magazine new series (Mayo et al., 1995). In addition to covering many of the principal players in the field of aroid research, Nicolson's paper dealt with the evolution of family concepts and gave a comparison of the then current modern systems of classification. The papers by Mayo, Bogner and Boyce were more comprehensive in scope than that of Nicolson but still did not cover in great detail many of the participants in Araceae research. In contrast, this paper will cover all systematic and floristic work that deals with Araceae which is known to me. It will not, in general, deal with agronomic papers on Araceae such as the rich literature on taro and its cultivation, nor will it deal with smaller papers of a technical nature or those dealing with pollination biology. -
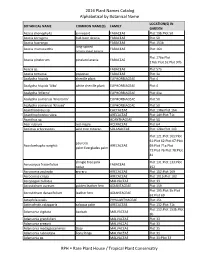
2016 Plant Names Catalog Alphabetical by Botanical Name
2016 Plant Names Catalog Alphabetical by Botanical Name LOCATION(S) IN BOTANICAL NAME COMMON NAME(S) FAMILY GARDEN Acacia choriophylla cinnecord FABACEAE Plot 19b:Plot 50 Acacia cornigera bull-horn Acacia FABACEAE Plot 50 Acacia huarango FABACEAE Plot 153b long-spined Acacia macracantha FABACEAE Plot 164 acacia:steel Acacia Plot 176a:Plot Acacia pinetorum pineland acacia FABACEAE 176b:Plot 3a:Plot 97b Acacia sp. FABACEAE Plot 57a Acacia tortuosa poponax FABACEAE Plot 3a Acalypha hispida chenille plant EUPHORBIACEAE Plot 4 Acalypha hispida 'Alba' white chenille plant EUPHORBIACEAE Plot 4 Acalypha 'Inferno' EUPHORBIACEAE Plot 41a Acalypha siamensis 'Firestorm' EUPHORBIACEAE Plot 50 Acalypha siamensis 'Kilauea' EUPHORBIACEAE Plot 50 Acanthocereus sp. CACTACEAE Plot 138a:Plot 164 Acanthophoenix rubra ARECACEAE Plot 149:Plot 71c Acanthus sp. ACANTHACEAE Plot 50 Acer rubrum red maple ACERACEAE Plot 64 Acnistus arborescens wild tree tobacco SOLANACEAE Plot 128a:Plot 143 Plot 121:Plot 161:Plot 61:Plot 62:Plot 67:Plot paurotis Acoelorrhaphe wrightii ARECACEAE 69:Plot 71a:Plot palm:Everglades palm 72:Plot 76:Plot 78:Plot 81 shingle tree:pink Plot 131:Plot 133:Plot Acrocarpus fraxinifolius FABACEAE cedar 152 Acrocomia aculeata gru-gru ARECACEAE Plot 102:Plot 169 Acrocomia crispa ARECACEAE Plot 101b:Plot 102 Acropogon bullatus MALVACEAE Plot 33 Acrostichum aureum golden leather fern ADIANTACEAE Plot 159 Plot 195:Plot 3b:Plot Acrostichum danaeifolium leather fern ADIANTACEAE 63:Plot 69 Actephila ovalis PHYLLANTHACEAE Plot 151 Actinorhytis calapparia calappa palm ARECACEAE Plot 132:Plot 71c Plot 112:Plot 153b:Plot Adansonia digitata baobab MALVACEAE 3b Adansonia grandidieri MALVACEAE Plot 33 Adansonia gregorii MALVACEAE Plot 33 Adansonia madagascariensis Bozy MALVACEAE Plot 35 Adansonia rubrostipa Fony:Ringy MALVACEAE Plot 31 Adansonia sp. -

On Anthurium for Market Access
TTHHEE MMIINNIISSTTRRYY OOFF AAGGRRIICCUULLTTUURREE AANNDD AAGGRROO--BBAASSEEDD IINNDDUUSSTTRRYY THE MINISTRY OF AGRICULTURE AND AGRO-BASED INDUSTRY KKKUUUAAALLLAAA LLLUUUMMMPPPUUURRR MMMAAALLLAAAYYYSSSIIIAAA FFOORR MMAARRKKEETT AACCCCEESSSS OONN AANNTTHHUURRIIUUMM CCCRRROOOPPP PPPRRROOOTTTEEECCCTTTIIIOOONNN&&&PPPLLLAAANNNTTTQQQUUUAAARRRAAANNNTTTIIINNNEEESSSEEERRRVVVIIICCCEEESSSDDDIIIVVVIIISSSIIIOOONNN DDDEEEPPPAAARRRTTTMMMEEENNNTTT OOOFFF AAAGGGRRRIIICCCUUULLLTTTUUURRREEE KKKUUUAAALLLAAA LLLUUUMMMPPPUUURRR MMMAAALLLAAAYYYSSSIIIAAA 222000000444 Technical Docu ment For Market Access On Anthurium Page i Ms. Asna Booty Othman, Director, Crop Protection and Plant Quarantine Services Division, Department of Agriculture Malaysia, wishes to extend her appreciation and gratitude to the following for their contribution, assistance and cooperation in the preparation of this Technical Document For Anthurium:- Mr. Muhamad Hj. Omar, Assistant Director, Phytosanitary and Export Control Section, Crop Protection and Plant Quarantine Services Division, Department of Agriculture Malaysia; Ms. Nuraizah Hashim, Agriculture Officer, Phytosanitary and Export Control Section, Crop Protection and Plant Quarantine Services Division, Department of Agriculture Malaysia; Mr. Tung Khoon Cheong, Assistant Director, Industrial Crop and Horticulture Division, Department of Agriculture Malaysia. Appreciation is also extended to Y. Bhg. Dato’ Ismail Ibrahim, Director- General of Agriculture, for his support and guidance in the preparation of this Document. Technical Document -

Many Independent Origins of Trans Splicing of a Plant Mitochondrial Group II Intron
J Mol Evol (2004) 59:80–89 DOI: 10.1007/s00239-004-2606-y Many Independent Origins of trans Splicing of a Plant Mitochondrial Group II Intron Yin-Long Qiu,1,2 Jeffrey D. Palmer1 1 Department of Biology, Indiana University, Bloomington, IN 47405, USA 2 Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, MI 48109, USA Received: 1 October 2003 / Accepted: 6 February 2004 Abstract. We examined the cis- vs. trans-splicing Introduction status of the mitochondrial group II intron nad1i728 in 439 species (427 genera) of land plants, using both The trans splicing of group II introns, i.e., fragmen- Southern hybridization results (for 416 species) and tation of an intron into at least two separate pieces intron sequence data from the literature. A total of that belong to different transcriptional units and 164 species (157 genera), all angiosperms, was found splicing of these intron pieces that are reunited at the to have a trans-spliced form of the intron. Using a RNA level via intermolecular base pairing, was first multigene land plant phylogeny, we infer that the discovered in the chloroplast genes rps12 (Fukuzawa intron underwent a transition from cis to trans et al. 1986; Koller et al. 1987; Zaita et al. 1987) and splicing 15 times among the sampled angiosperms. In psaA (Ku¨ ck et al. 1987). A few years later, several 10 cases, the intron was fractured between its 5¢ end research groups reported a total of six trans-spliced and the intron-encoded matR gene, while in the other introns in the angiosperm mitochondrial genes nad1 5 cases the fracture occurred between matR and the 3¢ (Chapdelaine and Bonen 1991; Conklin et al. -

Philodendron Schott (Araceae): Systematics and Evolution of a Mega-Diverse Genus from the New World
Philodendron Schott (Araceae): Systematics and evolution of a mega-diverse genus from the New World Inaugural-Dissertation to obtain the academic degree Doctor rerum naturalium (Dr. rer. nat.) submitted to the Department of Biology, Chemistry and Pharmacy of Freie Universität Berlin by Dubán Canal Gallego 2018 1st reviewer: Prof. Dr. Thomas Borsch 2nd reviewer: Prof. Dr. Julien Bachelier Date of disputation: Wednesday, 13th February 2019 ii “Somewhere, something incredible is waiting to be known” Carl Sagan (1934 -1996) iii Acknowledgements Acknowledgements First of all, I am honored to have worked under the supervision of Prof. Dr. Thomas Borsch who provided me the opportunity of conducting this investigation at the Botanischer Garten und Botanisches Museum Berlin - BGBM. Prof. Borsch helped me to design my project and guided me through my studies. I thank Prof. Dr. Julien Bachelier for taking over the role as second reviewer. I am deeply grateful to Dr. Nils Köster, who shared his endless passion for aroids and encouraged me during my studies. Nils joined me during field work in Colombia, gathered a large part of the samples used in this study, introduced me into the Araceae collections at BGBM and discussed various taxonomic issues with me to ensure the correct identification of the respective plants. I further deeply acknowledge the support of Dr. Katy E. Jones during the elaboration of my all analyses. Her discipline, respect and positive attitude inspired me. Katy made me think that science can be surprisingly easy. I especially thank Dr. Nadja Korotkova for her careful revision of my manuscripts and for supporting me all the way up to the submission of my thesis.