Alacepril a Neuronal Positive Control
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Key Enzymes Involved in the Synthesis of Hops Phytochemical Compounds: from Structure, Functions to Applications
International Journal of Molecular Sciences Review Key Enzymes Involved in the Synthesis of Hops Phytochemical Compounds: From Structure, Functions to Applications Kai Hong , Limin Wang, Agbaka Johnpaul , Chenyan Lv * and Changwei Ma * College of Food Science and Nutritional Engineering, China Agricultural University, 17 Qinghua Donglu Road, Haidian District, Beijing 100083, China; [email protected] (K.H.); [email protected] (L.W.); [email protected] (A.J.) * Correspondence: [email protected] (C.L.); [email protected] (C.M.); Tel./Fax: +86-10-62737643 (C.M.) Abstract: Humulus lupulus L. is an essential source of aroma compounds, hop bitter acids, and xanthohumol derivatives mainly exploited as flavourings in beer brewing and with demonstrated potential for the treatment of certain diseases. To acquire a comprehensive understanding of the biosynthesis of these compounds, the primary enzymes involved in the three major pathways of hops’ phytochemical composition are herein critically summarized. Hops’ phytochemical components impart bitterness, aroma, and antioxidant activity to beers. The biosynthesis pathways have been extensively studied and enzymes play essential roles in the processes. Here, we introduced the enzymes involved in the biosynthesis of hop bitter acids, monoterpenes and xanthohumol deriva- tives, including the branched-chain aminotransferase (BCAT), branched-chain keto-acid dehydroge- nase (BCKDH), carboxyl CoA ligase (CCL), valerophenone synthase (VPS), prenyltransferase (PT), 1-deoxyxylulose-5-phosphate synthase (DXS), 4-hydroxy-3-methylbut-2-enyl diphosphate reductase (HDR), Geranyl diphosphate synthase (GPPS), monoterpene synthase enzymes (MTS), cinnamate Citation: Hong, K.; Wang, L.; 4-hydroxylase (C4H), chalcone synthase (CHS_H1), chalcone isomerase (CHI)-like proteins (CHIL), Johnpaul, A.; Lv, C.; Ma, C. -

Supplementary Appendix 1. Search Strategy for the Systematic Review and Meta-Analysis
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Thorax Supplementary Appendix 1. Search strategy for the systematic review and meta-analysis # COVID-19 AND (ACEI or ARB) Pubmed #1. COVID-19 ((((novel[Title/Abstract]) AND (((corona[Title/Abstract]) AND virus[Title/Abstract]) OR (coronavirus[Title/Abstract]))) OR ((COVID[Title/Abstract]) OR (COVID-19[Title/Abstract]) OR (nCoV[Title/Abstract]) OR (2019-nCoV[Title/Abstract]) OR (Novel Coronavirus Pneumon.ia[Title/Abstract]) OR (NCP[Title/Abstract]) OR (severe acute respiratory infection[Title/Abstract]) OR (SARI[Title/Abstract]) OR (SARS-CoV-2[Title/Abstract]))) #2. ARB (("Angiotensin Receptor Antagonists"[Mesh]) OR (((angiotensin receptor blocker[Title/Abstract]) OR angiotensin receptor blockers[Title/Abstract]) OR ARB.*[Title/Abstract]) OR (((angiotensin[Title/Abstract]) AND receptor[Title/Abstract]) AND (antagonist.*[Title/Abstract] OR inhibitor.*[Title/Abstract] OR blocker.*[Title/Abstract]))) OR (ARB[Title/Abstract]) OR (olmesartan[Title/Abstract]) OR (valsartan[Title/Abstract]) OR (eprosartan[Title/Abstract]) OR (irbesartan[Title/Abstract]) OR (candesartan[Title/Abstract]) OR (losartan[Title/Abstract]) OR (telmisartan[Title/Abstract]) OR (azilsartan[Title/Abstract]) OR (tasosartan[Title/Abstract]) OR (embusartan[Title/Abstract]) OR (forasartan[Title/Abstract]) OR (milfasartan[Title/Abstract]) OR (saprisartan[Title/Abstract]) OR (zolasartan[Title/Abstract]) -

Beer As a Source of Hop Prenylated Flavonoids, Compounds with Antioxidant, Chemoprotective and Phytoestrogen Activity
BEER AS A SOURCE OF HOP PRENYLATED FLAVONOIDS, COMPOUNDS WITH ANTIOXIDANT, CHEMOPROTECTIVE AND PHYTOESTROGEN ACTIVITY Dana Urminská*1 and Nora Jedináková2 Address(es): Doc. RNDr. Dana Urminská, CSc., 1Slovak University of Agriculture, Faculty of Biotechnology and Food Sciences, Department of Biochemistry and Biotechnology, Trieda Andreja Hlinku 2, 949 76 Nitra-Chrenová, Slovakia, phone number: +421 37 641 4696. *Corresponding author: [email protected] https://doi.org/10.15414/jmbfs.4426 ARTICLE INFO ABSTRACT Received 4. 3. 2021 Beer is an alcoholic beverage consumed worldwide, which is given its typical taste by the presence of hops. Hops contain an array Revised 1. 6. 2021 technologically important substances, which are primarily represented by hop resins (humulones, lupulones, humulinones, hulupones, Accepted 1. 6. 2021 etc.), essential oils (humulene, myrcene, etc.) and tannins (phenolic compounds quercetin, catechin, etc.). In addition to their sensory Published 1. 8. 2021 properties, these molecules contribute to the biological and colloidal stability of beer with their antiseptic and antioxidant properties. Recently, an increased attention has been given to prenylated hop flavonoids, particularly xanthohumol, isoxanthohumol and 8- prenylnaringenin. Xanthohumol is a prenylated chalcone that exhibit antioxidant, anticancer and chemoprotective effects. Its only source Regular article in human nutrition is beer, nevertheless a large part of xanthohumol from hops is isomerized by heat to isoxanthohumol and desmethylxanthohumol, from which a racemic mixture of 6- and 8-prenylnaringenins is formed during the beer production. Xanthohumol is also converted to isoxanthohumol by digestion, leading to the formation of 8-prenylnaringenin that is being catalyzed by the enzymes of the intestinal microorganisms as well as liver enzymes. -

Epidemiology of Depressive Disorders 21 Aetiology 22 Treatment of Depressive Disorders 40 Prognosis 51
The assessment of serotonin function in major depression MD Thesis William Richard Whale 2005 1 UMI Number: U201052 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. Dissertation Publishing UMI U201052 Published by ProQuest LLC 2014. Copyright in the Dissertation held by the Author. Microform Edition © ProQuest LLC. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 Abstract Major depression is a common psychiatric disorder with considerable associated morbidity and mortality. Investigations to understand the causes of depression and how it can be more effectively treated are high priority. The serotonergic system has been implicated in the aetiology and treatment of depression by a wealth of preclinical and clinical evidence. This includes findings that drugs increasing serotonin neurotransmission have antidepressant action and inhibition of serotonin synthesis (via tryptophan depletion) can induce relapse of symptoms in depressed patients. Neuroendocrine challenges are an established method of indirectly examining brain serotonergic function, utilising changes in the secretion of pituitary hormones influenced by tonic serotonergic activity at the level of the hypothalamus. This thesis describes the development of two such neuroendocrine challenge tests, using the selective serotonergic probes, zolmitriptan and citalopram. Orally administered zolmitriptan, licensed for the treatment of migraine, clearly elevated plasma growth hormone in healthy subjects. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0121040 A1 Forster Et Al
US 20040121040A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0121040 A1 Forster et al. (43) Pub. Date: Jun. 24, 2004 (54) METHOD OF PRODUCING A (21) Appl. No.: 10/724,237 XANTHOHUMOL-CONCENTRATED HOP EXTRACTED AND USE THEREOF (22) Filed: Dec. 1, 2003 (75) Inventors: Adrian Forster, Wolnzach (DE); Josef (30) Foreign Application Priority Data Schulmeyr, Wolnzach (DE); Roland Schmidt, Wolnzach (DE); Karin Nov. 30, 2002 (DE)..................................... 102 56 O31.5 Simon, Pfaffenhofen (DE); Martin O O Ketterer, Wolnzach (DE); Birgit Publication Classification Forchhammer, Neustadt (DE); Stefan 7 Geyer, Wolnzach (DE); Manfred s - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - close Gehrig, Woln Zach (DE) ( ) O O - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - f Correspondence Address: (57) ABSTRACT BROWDY AND NEIMARK, P.L.L.C. 624 NINTH STREET, NW SUTE 300 In a method of producing a Xanthohumol-concentrated hop WASHINGTON, DC 20001-5303 (US) extract, the Xanthohumol-containing hop extract is extracted from a Xanthohumol-containing hop raw material by highly (73) Assignee: NATECO2 GMBH & CO. KG, Woln compressed CO as a solvent at pressures above 500 bar and Zach (DE) temperatures above 60° C. US 2004/O121040 A1 Jun. 24, 2004 METHOD OF PRODUCING A the finished beer or other kinds of food or used by itself as XANTHOHUMOL-CONCENTRATED HOP a chemo-preventive preparation. EXTRACTED AND USE THEREOF 0010 Presently, there are two prior art solutions. DE 199 39350 A1 describes a method of producing a xanthohumol BACKGROUND OF THE INVENTION enriched hop extract, with combinations of water and etha nol being used preferably in two steps of extraction. 5 to 15 0001) 1. Field of the Invention percent by weight of Xanthohumol are specified as being 0002 The invention relates to a method of producing a typical. -

Xanthohumol, a Prenylated Chalcone Derived from Hops, Inhibits Growth and Metastasis of Melanoma Cells
cancers Article Xanthohumol, a Prenylated Chalcone Derived from Hops, Inhibits Growth and Metastasis of Melanoma Cells Tatjana Seitz 1,2, Christina Hackl 3, Kim Freese 1, Peter Dietrich 1,4 , Abdo Mahli 1 , Reinhard Manfred Thasler 5, Wolfgang Erwin Thasler 6, Sven Arke Lang 7, Anja Katrin Bosserhoff 1,8 and Claus Hellerbrand 1,2,8,* 1 Institute of Biochemistry (Emil-Fischer-Zentrum), Friedrich-Alexander University Erlangen-Nürnberg, D-91054 Erlangen, Germany; [email protected] (T.S.); [email protected] (K.F.); [email protected] (P.D.); [email protected] (A.M.); [email protected] (A.K.B.) 2 Department of Internal Medicine I, University Hospital Regensburg, D-93053 Regensburg, Germany 3 Department of Surgery, University Hospital Regensburg, D-93053 Regensburg, Germany; [email protected] 4 Medical Clinic 1, Department of Medicine, University Hospital Erlangen, Friedrich-Alexander-University, D-91054 Erlangen, Germany 5 Stiftung HTCR, BioPark III, Am BioPark 13, D-93053 Regensburg, Germany; [email protected] 6 Hepacult GmbH, D-82152 Martinsried, Germany; [email protected] 7 Department of Surgery and Transplantation, University Hospital RWTH Aachen, D-52074 Aachen, Germany; [email protected] 8 Comprehensive Cancer Center (CCC) Erlangen-EMN, D-91054 Erlangen, Germany * Correspondence: [email protected] Simple Summary: Melanoma is an aggressively growing form of skin cancer. It has a high metastatic Citation: Seitz, T.; Hackl, C.; potential, and the liver is one of the most common sites for visceral metastasis. Patients with Freese, K.; Dietrich, P.; Mahli, A.; hepatic metastases have a very poor prognosis, and effective forms of treatment are urgently needed. -

WO 2017/055924 A2 6 April 2017 (06.04.2017) W P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/055924 A2 6 April 2017 (06.04.2017) W P O PCT (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 33/04 (2006.01) A61K 9/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/IB2016/0015 10 KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (22) International Filing Date: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 28 September 2016 (28.09.201 6) OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (25) Filing Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (26) Publication Language: English ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 62/233,906 28 September 2015 (28.09.2015) US kind of regional protection available): ARIPO (BW, GH, 62/233,941 28 September 2015 (28.09.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: M.G. -

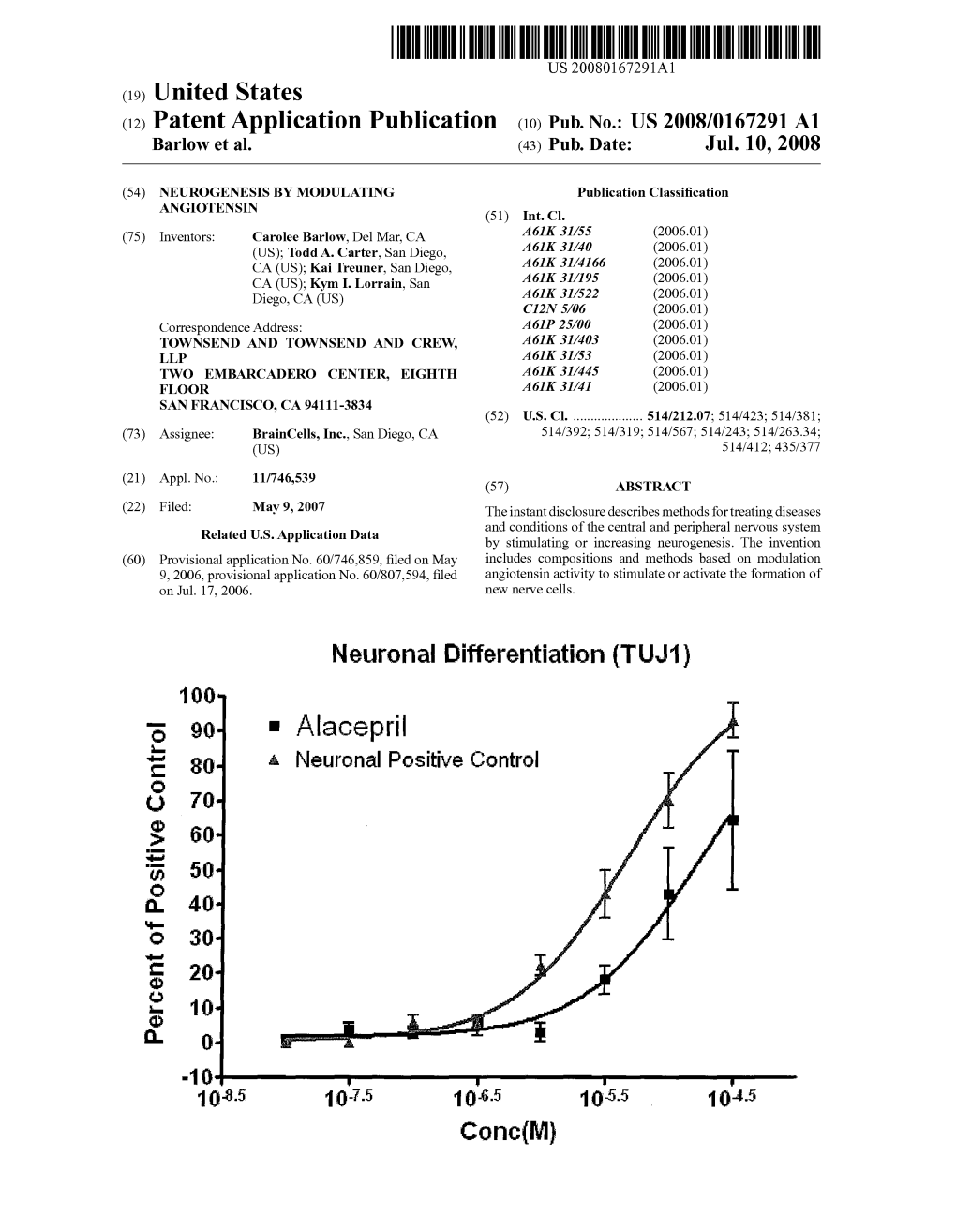
Alacepril Tablet 6 Mg Alacepril Tablet 12.5 Mg Alacepril Tablet 25 Mg
The following is an English translation of the package insert for the drug sold in Japan (as of December 2012). Chronic Heart Failure Drug for Dogs Veterinary Drug Prescription Legend Drug Alacepril Tablet 6 mg Alacepril Tablet 12.5 mg Alacepril Tablet 25 mg Alacepril Tablet is a mild, long-acting angiotensin-converting enzyme (ACE, kininase II) inhibitor synthesized and developed by Dainippon Sumitomo Pharma Co., Ltd. The active ingredient alacepril is converted in the body to deacetylalacepril, which is then further metabolized to captopril. Deacetylalacepril is effectively delivered to the arterial wall, where it directly inhibits peripheral sympathetic activity, resulting in vasodilation without modulation of the renin-angiotensin-aldosterone (RAA) system. In addition, the active metabolite captopril inhibits ACE, thereby producing both arterial and venous dilation through modulation of the RAA system. In an experimental chronic heart failure model in dogs, a single dose of 1 to 3 mg of alacepril was shown to result in sustained reduction of preload and afterload. In clinical field studies, Alacepril Tablet was administered as a single agent or in combination with diuretic or cardiotonic agents to dogs with chronic heart failure from mitral regurgitation. The results showed that Alacepril Tablet more effectively inhibited the progression of heart disease and increased the exercise tolerance of animals when compared to treatment with conventional diuretic and cardiotonic agents alone, thereby demonstrating that Alacepril Tablet produces a significant improvement in symptoms. Composition Alacepril Tablet 6 mg contains 6 mg of alacepril per tablet. Alacepril Tablet 12.5 mg contains 12.5 mg of alacepril per tablet. -

Modifications to the Harmonized Tariff Schedule of the United States To
U.S. International Trade Commission COMMISSIONERS Shara L. Aranoff, Chairman Daniel R. Pearson, Vice Chairman Deanna Tanner Okun Charlotte R. Lane Irving A. Williamson Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement the Dominican Republic- Central America-United States Free Trade Agreement With Respect to Costa Rica Publication 4038 December 2008 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 18, 2008, set forth in the Appendix hereto, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement the Dominican Republic- Central America-United States Free Trade Agreement, as approved in the Dominican Republic-Central America- United States Free Trade Agreement Implementation Act, with respect to Costa Rica. (This page is intentionally blank) Annex I Effective with respect to goods that are entered, or withdrawn from warehouse for consumption, on or after January 1, 2009, the Harmonized Tariff Schedule of the United States (HTS) is modified as provided herein, with bracketed matter included to assist in the understanding of proclaimed modifications. The following supersedes matter now in the HTS. (1). General note 4 is modified as follows: (a). by deleting from subdivision (a) the following country from the enumeration of independent beneficiary developing countries: Costa Rica (b). -

Pdf4 Complex I and the Aryl Palladium Precursor II Underwent Sequential Single Electron Abstraction from Aryl Pd(II) Complex
Design, synthesis, methodology development, and evaluation of PET imaging agents targeting cancer and CNS disorders By Gengyang Yuan B.S. in Chemical Engineering and Technology, Zhejiang University of Technology M.S. in Pharmaceutical Engineering, Zhejiang University A dissertation submitted to The Faculty of the College of Science of Northeastern University in partial fulfillment of the requirements for the degree of Doctor of Philosophy April 21, 2017 Dissertation directed by Michael P. Pollastri Associate Professor and Chair of Chemistry and Chemical Biology Co-directed by Neil Vasdev Adjunct Associate Professor of Chemsitry and Chemical Biology Associate Professor of Radiology, Massachusetts General Hospital and Harvard Medical School Dedication To my parents Zhijun and Yongmian and my wife Ran and daughter Isabella ii Acknowledgements This dissertation would not have been possible without the support, guidance and encouragement of numerous people who have helped me along the way. First and foremost, I would like to thank Northeastern University and the Department of Chemistry and Chemical Biology for supporting me to pursue my doctoral study. I would like to especially thank my current advisor Professor Michael Pollastri for helping me out when I needed it the most. I appreciate you for taking me into your group and giving me full support to finish my thesis projects. I also especially thank my co-advisor Professor Neil Vasdev for taking me into his group at Mass. General Hospital & Harvard Medical School and teaching me the PET radiochemistry and PET imaging. I could not image how I could accomplish this work without your help. I also got a lot of help from Dr. -

Angiotensin-Converting Enzyme (ACE)
Angiotensin-converting Enzyme (ACE) Angiotensin-converting enzyme (ACE) indirectly increases blood pressure by causing blood vessels to constrict. ACE does that by converting angiotensin I to angiotensin II, which constricts the vessels. ACE, angiotensin I and angiotensin II are part of the renin-angiotensin system (RAS), which controls blood pressure by regulating the volume of fluids in the body. ACE is secreted in the lungs and kidneys by cells in the endothelium (inner layer) of blood vessels. It has two primary functions: ACE catalyses the conversion of angiotensin I to angiotensin II, a potent vasoconstrictor in a substrate concentration-dependent manner. ACE degrades bradykinin, a potent vasodilator, and other vasoactive peptides. These two actions make ACE inhibition a goal in the treatment of conditions such as high blood pressure, heart failure, diabetic nephropathy, and type 2 diabetes mellitus. Inhibition of ACE (by ACE inhibitors) results in the decreased formation of angiotensin II and decreased metabolism of bradykinin, leading to systematic dilation of the arteries and veins and a decrease in arterial blood pressure. www.MedChemExpress.com 1 Angiotensin-converting Enzyme (ACE) Inhibitors & Activators (R)-MLN-4760 Alamandine Cat. No.: HY-19414A Cat. No.: HY-P3108 (R)-MLN-4760, the R-enantiomer of MLN-4760, is an Alamandine, a member of the renin-angiotensin ACE2 inhibitor, with an IC50 of 8.4 μM. system (RAS), a vasoactive peptide, is an (R)-MLN-4760 is the less active isomer. endogenous ligand of the G protein-coupled receptor MrgD. Alamandine targets to protect the kidney and heart through anti-hypertensive actions. Purity: >98% Purity: 98.95% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 1 mg, 5 mg, 10 mg, 25 mg Size: 5 mg Angiotensin (1-7) Angiotensin (1-7) (acetate) (Ang-(1-7)) Cat. -

Angiotensin Converting Enzyme Inhibitors in the Treatment of Hypertension
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Publications of the IAS Fellows CHEMISTRY – STRUCTURE, SYNTHESIS AND DYNAMICS Angiotensin converting enzyme inhibitors in the treatment of hypertension Bhaskar J. Bhuyan and Govindasamy Mugesh* Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bangalore 560 012, India verting enzyme’ (ACE). Further, ACE is also responsible Angiotensin converting enzyme (ACE) catalyses the conversion of angiotensin I (Ang I) to angiotensin II for the elevation of BP by cleaving the terminal dipeptide (Phe–Arg) of vasodilator hormone bradykinin to its (Ang II). The ACE activity directly related to hyperten- 5,6 sion as Ang II is the blood pressure regulating hormone. inactive form (bradykinin 1–7, Figure 1) . Therefore, ACE inhibitors are a major class of anti- Involvement of RAS in elevation of BP was reported hypertensive drugs. Captopril, chemical name, was for the first time by Tigerstedt and Bergman7, who have the first orally active ACE inhibitory antihypertensive shown that the saline extract of kidney contains some drug, discovered in 1977. Since then, a number of such vasopressor (material that increases BP) activity. It was drugs have been synthesized. Enzyme-inhibitor bound named ‘renin’ as it was extracted from kidney. In 1940s, crystal structural studies reveal a great deal of under- Braun-Mendez and co-workers8 discovered that renin standing about the interactions of the inhibitors at the catalyses the formation of the actual pressor agent, the active site of ACE. This can be helpful in the rational ‘angiotensinogen’ (also called hypertensinogen).