Soil Carbon Dynamics in the Upper Mersey Estuarine Floodplain, Northwest England: Implications for Soil Carbon Sequestration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lepidopterous Fauna Lancashire and Cheshire
LANCASHIRE AND CHESHIRE LEPIDOPTERA, THE LEPIDOPTEROUS FAUNA OF LANCASHIRE AND CHESHIRE COMPILED BY WM. MANSBRIDGE, F.E.S., Hon. Sec. La11c:1 shire and Cheshire Entomological Society. BEING A NEW EDITION OF Dr. ELLIS'S LIST brought up to date with the a~s istance of the Lepidoptcrists whose names nppcnr below. Ark le, J., Chester A. Baxter, T., Min-y-don, St. Annes-on-Sea T.B. Bell, Dr. Wm., J.P., Rutland House, New Brighton W.B. Boyd, A. W., M.A., F.E.S., The Alton, Altrincham ... A.W.B Brockholes, J. F. The late J.F.B. Capper, S. J. The late .. S.J.C. Chappell, Jos. The late .. J C. Collins, Joseph, The University Museum, Oxford J. Coll. Cooke, N. The late N.C. Corbett, H. H., Doncaster H.H.C. Cotton, J., M.R.C.S., etc., Simonswood, Prescot Rd., St. Helens ... ]. Cot. Crabtree, B. H., F. E.S., Cringle Lodge, Leve nshulme, Manchester ... B.H.C. Day, G. 0 ., F.E.S. late of Knutsforcl ... D. Wolley-Dod, F. H, Edge, near Malpas F.H.W.D. Ellis, John W ., M.B. (Vic), F.E.S., etc., 18, Rodney Street, Liverpool J.W.E. Forsythe, Claude F., The County Asylum, Lancaster C.H F. Frewin, Colonel, Tarvin Sands ... F. Greening, Noah, The late N.G. Gregson, Chas. S., The late C.S.G. Gregson, W., The late ... W.G. Harrison, Albert, F.E.S., The lalt1 A.H. 2 LANCASHIRE AND CHESHIRE LEPIDOPTERA. LANCASHIRE AND CHESHIRE LEPIDOPTERA. 3 Harrison, W. W.H. Higgins, Rev: H. -

Historical Group
Historical Group NEWSLETTER and SUMMARY OF PAPERS No. 64 Summer 2013 Registered Charity No. 207890 COMMITTEE Chairman: Prof A T Dronsfield | Prof J Betteridge (Twickenham, 4, Harpole Close, Swanwick, Derbyshire, | Middlesex) DE55 1EW | Dr N G Coley (Open University) [e-mail [email protected]] | Dr C J Cooksey (Watford, Secretary: Prof. J. W. Nicholson | Hertfordshire) School of Sport, Health and Applied Science, | Prof E Homburg (University of St Mary's University College, Waldegrave | Maastricht) Road, Twickenham, Middlesex, TW1 4SX | Prof F James (Royal Institution) [e-mail: [email protected]] | Dr D Leaback (Biolink Technology) Membership Prof W P Griffith | Dr P J T Morris (Science Museum) Secretary: Department of Chemistry, Imperial College, | Mr P N Reed (Steensbridge, South Kensington, London, SW7 2AZ | Herefordshire) [e-mail [email protected]] | Dr V Quirke (Oxford Brookes Treasurer: Dr J A Hudson | University) Graythwaite, Loweswater, Cockermouth, | Prof. H. Rzepa (Imperial College) Cumbria, CA13 0SU | Dr. A Sella (University College) [e-mail [email protected]] Newsletter Dr A Simmons Editor Epsom Lodge, La Grande Route de St Jean, St John, Jersey, JE3 4FL [e-mail [email protected]] Newsletter Dr G P Moss Production: School of Biological and Chemical Sciences, Queen Mary University of London, Mile End Road, London E1 4NS [e-mail [email protected]] http://www.chem.qmul.ac.uk/rschg/ http://www.rsc.org/membership/networking/interestgroups/historical/index.asp 1 RSC Historical Group Newsletter No. 64 Summer 2013 Contents From the Editor 2 Obituaries 3 Professor Colin Russell (1928-2013) Peter J.T. -

MERSEY GATEWAY ENVIRONMENTAL TRUST Local Resident Contact Details: Honorary Vice President of Welcome to the Mersey Gateway Environmental Trust (MGET)
Meet the MGET team Our measure for success The Trust’s Board of directors consists of: For the MGET to be a success we need results • 2 elected councillors from Halton Borough Council on the ground. We aim to: and Warrington Council 1. Create a 28.5 hectare nature reserve running • 2 elected councillors from parish councils in Halton 200m either side of the Mersey Gateway. and Warrington. Currently there is one parish council 2. Ensure that an area of 1654 hectares is vacancy. recognised as an enjoyable place to visit that • 2 local residents. people can be proud of. 3. Bring saltmarsh and reedbeds back into Cllr. Keith Morley management. Represents: 4. Increase bird numbers with accurate and Broadheath ward, Widnes. regular monitoring. 5. Generate substantial new funding to come into the area. Yousuf Shaikh Chair of Walton Parish Council, Warrington. Parish Cllr. Researchers from the University of Salford Cllr. Geoff Settle Want to learn more? Represents: Poulton North ward, Warrington, Steering Group Member Mersey Along with our regular newsletter, look out for Forest, Chair Warrington Nature our updates online at www.merseygateway.co.uk Conservation Forum and follow our environmental activities on Twitter @merseygateway Professor David Norman MERSEY GATEWAY ENVIRONMENTAL TRUST Local resident Contact details: Honorary vice president of Welcome to the Mersey Gateway Environmental Trust (MGET). We’ve been set-up to deliver lasting Cheshire Wildlife Trust and Paul Oldfield environmental benefits associated with the Mersey Gateway, a nationally important new bridge crossing author of Birds of Cheshire. Company Secretary over the Mersey Estuary between Runcorn and Widnes. -

Tam O'shanter Urban Farm Management Plan 2007 – 2012
Tam O’Shanter Urban Farm Management Plan 2007 – 2012 Contents 1) Introduction and vision 3 2) Site Description 4 4) Analysis and assessment including Security Audit 13 5) Strategic Aims and Objectives 22 6) Action plan 37 7) Monitoring and review 41 Appendices; 1) Animal Welfare Policy 42 2) Volunteer Policy, Volunteer Fact Sheet and Application form 43 3) Farm Plan & aerial photograph 46 4) Emergency Procedure 48 5) Stocking Level 49 6) Five Year Budget 50 7) The Green Pennant Award 2006/2007 judging feedback 53 8) Security Audit 55 2 1) Introduction and vision This plan is intended to provide a framework for the development and improvement of the farm over the next five-year period up to 2011. The plan is intended to be a working document, which is open to new ideas at any time. We welcome your suggestions and comments for incorporation into this plan, whether you are a local resident, user or organisation. Your input will help us to develop a farm that meets everyone’s needs and aspirations. If you wish to find out further information about this document or submit any suggestions please contact the farm’s Manager John Jakeman on 0151 653 9332 or by email at [email protected]. Alternatively, you can contact John Jakeman by writing to: Tam O’Shanter Urban Farm, Boundary Road, Bidston, Wirral, CH43 7PD Vision: • To create an urban farm for educational, recreational and community use based at Tam O’Shanter Cottage, Bidston, Wirral. • To enhance Bidston Hill as a site for countryside recreation 3 2) Site Description Name: Tam O’Shanter -

WALK 1 Bidston Hill & River Fender
Information WALK 1 Bidston Hill & River Fender WALK 2 The Wonders of Birkenhead This Walk and Cycle leaflet for Wirral covers the north eastern quarter and is one of a series of A circular walk starting at the Tam O’Shanter 2a Turn left onto Noctorum Lane. Follow this grows in the shallow sandy soils. Follow the main path Birkenhead has some fascinating historical traffic lights and turn left into Ivy Street, following 7 From the Transport Museum retrace your steps four leaflets each consisting of three walks and Urban Farm, this route takes you across Wirral unsurfaced lane to the junction with Budworth Road. along this natural Sandstone Pavement. The Windmill attractions and if you haven’t yet discovered the Birkenhead Priory sign on your right. Birkenhead back to Pacific Road where there is the Pacific Road one cycle route. Cross with care as there is a blind bend to the right. should now be coming into view. Priory is at the end of Priory Street on the left. This Arts Centre and on towards the river to view the Ladies Golf Course, along the River Fender and Continue along Noctorum Lane past Mere Bank on the them you may be pleasantly surprised. This walk former Benedictine monastery has an exhibition and the is Mersey Tunnel Ventilation Tower. The architect who 8 Continue to the iron footbridge above the deep rocky I have recently updated all 12 walks based on back to the heights of Bidston Hill with views of right. Continue straight ahead. The track swings right visits ten of them. -

The Story of Our Lighthouses and Lightships
E-STORy-OF-OUR HTHOUSES'i AMLIGHTSHIPS BY. W DAMS BH THE STORY OF OUR LIGHTHOUSES LIGHTSHIPS Descriptive and Historical W. II. DAVENPORT ADAMS THOMAS NELSON AND SONS London, Edinburgh, and Nnv York I/K Contents. I. LIGHTHOUSES OF ANTIQUITY, ... ... ... ... 9 II. LIGHTHOUSE ADMINISTRATION, ... ... ... ... 31 III. GEOGRAPHICAL DISTRIBUTION OP LIGHTHOUSES, ... ... 39 IV. THE ILLUMINATING APPARATUS OF LIGHTHOUSES, ... ... 46 V. LIGHTHOUSES OF ENGLAND AND SCOTLAND DESCRIBED, ... 73 VI. LIGHTHOUSES OF IRELAND DESCRIBED, ... ... ... 255 VII. SOME FRENCH LIGHTHOUSES, ... ... ... ... 288 VIII. LIGHTHOUSES OF THE UNITED STATES, ... ... ... 309 IX. LIGHTHOUSES IN OUR COLONIES AND DEPENDENCIES, ... 319 X. FLOATING LIGHTS, OR LIGHTSHIPS, ... ... ... 339 XI. LANDMARKS, BEACONS, BUOYS, AND FOG-SIGNALS, ... 355 XII. LIFE IN THE LIGHTHOUSE, ... ... ... 374 LIGHTHOUSES. CHAPTER I. LIGHTHOUSES OF ANTIQUITY. T)OPULARLY, the lighthouse seems to be looked A upon as a modern invention, and if we con- sider it in its present form, completeness, and efficiency, we shall be justified in limiting its history to the last centuries but as soon as men to down two ; began go to the sea in ships, they must also have begun to ex- perience the need of beacons to guide them into secure channels, and warn them from hidden dangers, and the pressure of this need would be stronger in the night even than in the day. So soon as a want is man's invention hastens to it and strongly felt, supply ; we may be sure, therefore, that in the very earliest ages of civilization lights of some kind or other were introduced for the benefit of the mariner. It may very well be that these, at first, would be nothing more than fires kindled on wave-washed promontories, 10 LIGHTHOUSES OF ANTIQUITY. -

New Brighton Kings Parade to Birkenhead Park
New Brighton Kings Parade to Birkenhead Park Walking & Cycling: Continue along the sea front walk and cycle track. When the two separate at the far end Derby Pool car park, walkers stay on the sea defence path. Cyclists can push their cycles along this section. Alternatively cyclists can follow the signs for the Wirral Circular Trail to the main Leasowe Road and turn right. This is a 40mph dual carriage-way with no specific cycle routes at present. Leasowe Castle is then on your right. Continue straight along, bearing right to the Lighthouse when the main road turns left. If you stay on the sea defence path, Leasowe Castle and then the Lighthouse are on your left. Driving: At the last roundabout on Kings Drive, turn left for the M53, then at the 2nd roundabout, take 1st left along Harrison Drive onto Wallasey Village and right at the roundabout for the A551, Leasowe Road. Follow this road, noting the bypass flyover, past Leasowe Castle on the right and then the Lighthouse ahead. Heritage Site 5 Leasowe Castle: Built by the Earls of Derby in the late 16th century, this Grade II* ‘Castle’ has been altered and enlarged over the centuries, serving among other things as a sporting lodge, a castellated mansion, an hotel, a nobleman’s residence and a railwayman’s convalescent home. Today it is once again a hotel. Leasowe Castle Heritage Site 6 Leasowe Lighthouse: The oldest brick-built lighthouse in Britain, it was erected in 1763 by the Liverpool Docks Committee. Originally it was one of four lights on the north coast of Wirral, the others being two at Hoylake and another - a lower light - at Leasowe. -
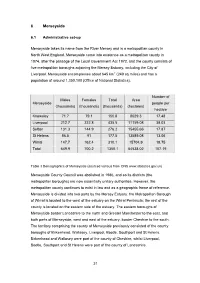
6 Merseyside
6 Merseyside 6.1 Administrative set-up Merseyside takes its name from the River Mersey and is a metropolitan county in North West England. Merseyside came into existence as a metropolitan county in 1974, after the passage of the Local Government Act 1972, and the county consists of five metropolitan boroughs adjoining the Mersey Estuary, including the City of Liverpool. Merseyside encompasses about 645 km2 (249 sq miles) and has a population of around 1,350,100 (Office of National Statistics). Number of Males Females Total Area Merseyside people per (thousands) (thousands) (thousands) (hectares) hectare Knowsley 71.7 79.1 150.8 8629.3 17.48 Liverpool 212.7 222.8 435.5 11159.08 39.03 Sefton 131.3 144.9 276.2 15455.66 17.87 St Helens 86.5 91 177.5 13589.08 13.06 Wirral 147.7 162.4 310.1 15704.9 19.75 Total 649.9 700.2 1350.1 64538.02 107.19 Table 3 Demographics of Merseyside (sourced various from ONS www.statistics.gov.uk) Merseyside County Council was abolished in 1986, and so its districts (the metropolitan boroughs) are now essentially unitary authorities. However, the metropolitan county continues to exist in law and as a geographic frame of reference. Merseyside is divided into two parts by the Mersey Estuary: the Metropolitan Borough of Wirral is located to the west of the estuary on the Wirral Peninsula; the rest of the county is located on the eastern side of the estuary. The eastern boroughs of Merseyside border Lancashire to the north and Greater Manchester to the east, and both parts of Merseyside, west and east of the estuary, border Cheshire to the south. -

Mersey Gateway Bridge Is a the Project Will Bring Huge Estimated Benefits for Cable-Stayed Structure with Three Pylons
Scheduled to open autumn 2017 The Mersey Gateway Project On track to open in autumn 2017, the Mersey Gateway You can now see detailed information about tolling at Project is a major civil engineering scheme to build a new www.merseyflow.co.uk. There are special discount deals six-lane cable-stayed toll bridge over the River Mersey and on tolls for eligible Halton residents and regular users along a 9.2 kilometre road network connecting the new bridge to with information about how blue badge holders can register the main motorway network in the north west of England. for free crossings. At a local level, the project will provide a much needed new Registration will open in the summer of 2017 but you can link between the towns of Runcorn and Widnes and will look online now to work out the best deal for you and see relieve the congested and ageing Silver Jubilee Bridge. what you need to do to register. The new bridge Benefits The design of the new Mersey Gateway Bridge is a The project will bring huge estimated benefits for cable-stayed structure with three pylons. people and businesses in Halton, the Liverpool city- region, Cheshire and across the north west. It will be 2.3km long with a river span of 1km. Up to 1,000 people are working on site on the project The main bridge deck is made from reinforced at any one time, and during the first year of construction concrete and the spans are supported by steel cable Merseylink issued contracts worth a total of £129 million stays attached to pylons rising up to between 80 and to north west businesses. -

A History of the Old Parish of Bidston, Cheshire
CHI'KCH KlIiSTON A HISTORY OF THE OLD PARISH OF BIDSTON, CHESHIRE. By John Brownbill, M.A. (Continued) CHAPTER VI. THE CLERGY AND THE CHARITIES. A CHURCH was probably built at Bidston, when the land was granted to the first Hamon de Mascy, and it appears to have been, granted, with Backford church, to the priory of Birkenheacl at its foundation. It is a pecu liarity that there is no glebe in the township of Bidston belonging to it, 1 and the reason may be that in founding and endowing the priory the Mascys intended that the monks should have entire charge of it, so that there the glebe was merged in the monastic estate. No vicarage was ever created, and there seems to have been no house for a resident priest. The monks had the tithes, and in 1291 the value of the church of Bedeston was £5 6s. 8d. 2 The old building having entirely disappeared, we must be content with the statement that it contained frag ments of Early English style ; there should have been traces of an even earlier church. The mediaeval history is a blank ; there is mention of the marriage of William Pulle and Isabel Boteler at the " parish kirk of Bidstone " in 1436. 3 The Valor Eccle- siasticus of 1534 gives a profit of 6s. 8d. from the glebe ; tithes of corn £7, Easter roll 405., small tithes 235., lambs 1 There is $ ac. in Claughton. 2 Tax. P. Nich., 248. 3 Child Alarriages (E.E. Text Soc.), p. Ixxxvii. This reference is due to Mr. -

The Marine Sale Marine The
Wednesday 18 April 2018 Wednesday THE MARINE SALE THE MARINE SALE | Knightsbridge, London | Wednesday 18 April 2018 24653 Bonhams 1793 Limited Bonhams International Board Bonhams UK Ltd Directors Registered No. 4326560 Robert Brooks Co-Chairman, Colin Sheaf Chairman, Gordon McFarlan, Andrew McKenzie, Registered Office: Montpelier Galleries Malcolm Barber Co-Chairman, Harvey Cammell Deputy Chairman, Simon Mitchell, Jeff Muse, Mike Neill, Montpelier Street, London SW7 1HH Colin Sheaf Deputy Chairman, Antony Bennett, Matthew Bradbury, Charlie O’Brien, Giles Peppiatt, India Phillips, Matthew Girling CEO, Lucinda Bredin, Simon Cottle, Andrew Currie, Peter Rees, John Sandon, Tim Schofield, +44 (0) 20 7393 3900 Patrick Meade Group Vice Chairman, Jean Ghika, Charles Graham-Campbell, Veronique Scorer, Robert Smith, James Stratton, +44 (0) 20 7393 3905 fax Jon Baddeley, Rupert Banner, Geoffrey Davies, Matthew Haley, Richard Harvey, Robin Hereford, Ralph Taylor, Charlie Thomas, David Williams, Jonathan Fairhurst, Asaph Hyman, James Knight, David Johnson, Charles Lanning, Grant Macdougall Michael Wynell-Mayow, Suzannah Yip. Caroline Oliphant, Shahin Virani, Edward Wilkinson, Leslie Wright. THE MARINE SALE Wednesday 18 April 2018 at 2pm Knightsbridge, London BONHAMS ENQUIRIES Please see page 2 for bidder IMPORTANT INFORMATION Montpelier Street information including after-sale In February 2014 the United Knightsbridge Pictures collection and shipment States Government announced London SW7 1HH Leo Webster the intention to ban the import www.bonhams.com +44 (0) 20 7393 3865 Please see back of catalogue of any ivory into the USA. Lots [email protected] for important notice to bidders containing ivory are indicated by VIEWING the symbol Ф printed beside the Sunday 15 April Veronique Scorer ILLUSTRATIONS Lot number in this catalogue. -

Ashdcrjvn FOREST Sussex Area
I GRADE 3 ASHDCrJVN FOREST Sussex 51/46-29- Area:1000 ha Altituder100-250m Geo1ogy:Wealden sandstone, peat I’ 0wners:Conservators of Ashdown Forest Total taxa: 24 terricolous i’I Scattered woodlands with open areas of heathland, very like the New Forest in character, provide a back-up to Ambersham in that series. The best areas are at Colemans Hatch (51/435326) and I km W. of the radio mast at Duddleswell (51/465293) but are small (4-5 ha). The most noteworthy lichens are Pycnothelia papillaria and Icmadophila ericetorum, Cladonia ciliata and C.strepsilis. Parts have been damaged by fire but are recovering and much remains intact. The site has been well studied in parts. It is under heavy visitor pressure. GRADE 3 LAVINCTON OMEMON Sussex 4 1/94-18- At ea: 20 ha Altitude: 30m Geo1ogy:Folkestone Sands 0wners:National Trust I! Total taxa: 55 Only about one quarter the size of AmbershamComnon with which it should be compared, the site is slightly less species rich. It is a back-up to that site, in the New Forest series‘. An area of Calluna dominated heathland between two woodland complexes, partly burned about 20 years ago, this site has a rich Cladonia i flora. While it is in danger from fire, it is well-protected and managed . Outstanding species include Cladonia sulphurina, C.rei and C.stre silis with Pycnothelia papillaria, C.arbuscula, C.bacilla- bdea- ol igotropha. The site is fairly well studied. I i! I 59 I i GRADE 3 SEVEN SISTERS Sussex 5015--9-- Area: 90ha Altitude: 30-75m Geo1ogy:Chalk Owners: Total taxa: 17 terricolous The site extends from Birling Gap to Cuckmere Haven and is some- what maritime in nature.